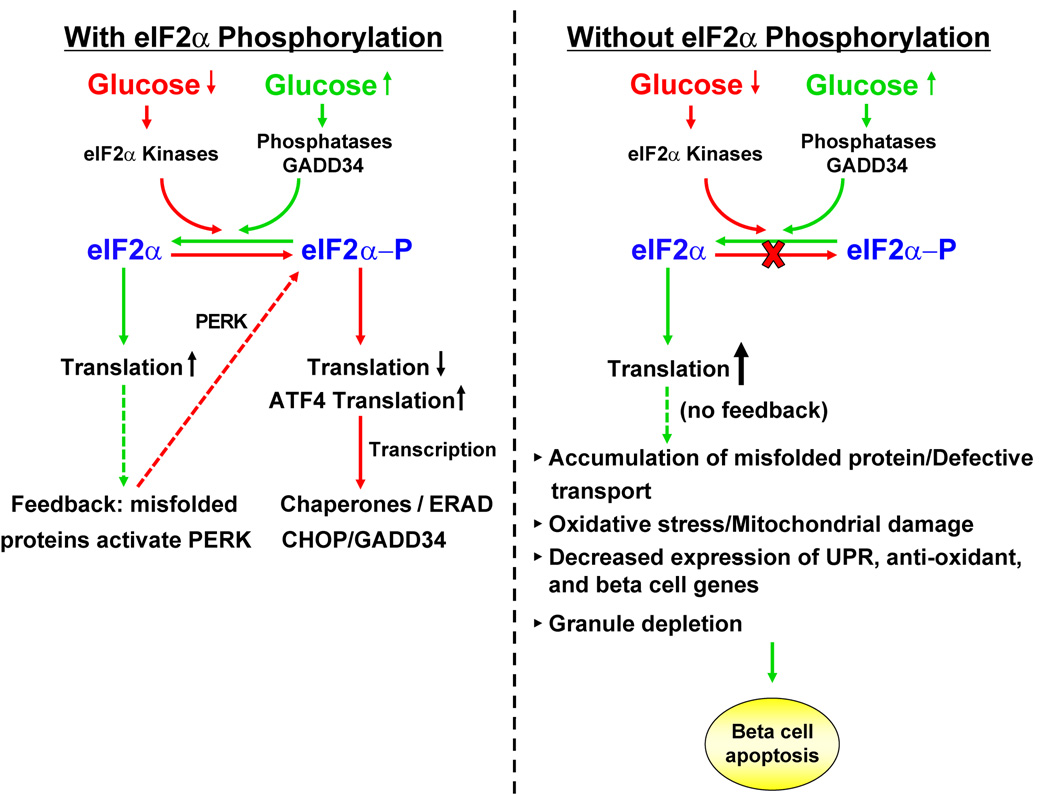
Figure 7. Mechanism for dysfunction and death of eIF2α phosphorylation-deficient beta cells.
(A) Fluctuations in glucose regulate eIF2α kinases and phosphatases to control eIF2α phosphorylation and the rate of mRNA translation in the beta cell. A feedback loop attenuates mRNA translation through PERK activation in response to misfolded proteins. Phosphorylation of eIF2α is required for translation of Atf4 mRNA that leads to transcriptional induction. Increased expression of GADD34 causes dephosphorylation of eIF2α to increase mRNA translation. The eIF2α A/A mutation prevents glucose repression of protein synthesis and causes unrestricted high rates of mRNA translation. Elevated protein synthesis leads to the accumulation of misfolded proteins in the ER. The absence of eIF2α phosphorylation reduces expression of UPR genes and antioxidant response genes, thereby exacerbating the protein-folding defect. Accumulation of misfolded proteins in the ER initiates production of ROS. ROS may inactivate beta cell-specific transcription factors, leading to insulin granule depletion. Beta cells ultimately succumb to apoptosis.