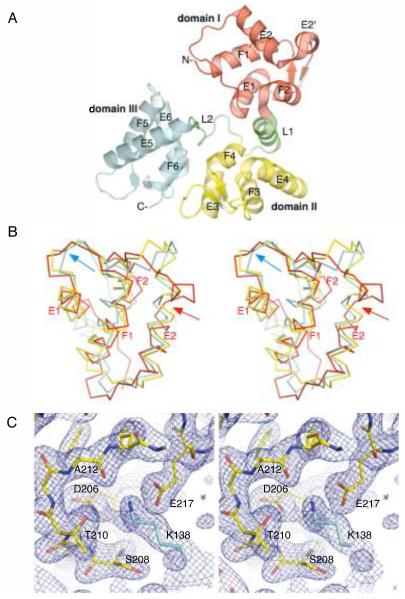
Figure 1.
The X-ray crystal structure of D.rerio secretagogin. (A) Secretagogin monomer consists of six EF-hand motifs arranged in pairs to form three globular domains (rendered consecutively in red, yellow and cyan). Linkers L1 and L2, highlighted in green, connect the individual domains. The apposed metal binding loops form antiparallel β-sheet on the outer surface of the V-shaped molecule. The helices of the individual EF-hand motifs are labeled for clarity. (B) A stereo view of Cα-trace of the superposed domains I, II and III (red, yellow and cyan, respectively) of secretagogin. The topologically equivalent motifs EF1, EF3 and EF5 overlap well, except for the Ca2+-binding loop which adopts the open, “Ca2+-ready” conformation in EF5-hand (cyan arrow) and closed, “Ca2+-free” conformation in EF1 and EF3 motifs. The EF2 hand differs from all the remaining EF-hands due to a break at Met63 in the helix E2 (red arrow). (C) A stereo image of the calcium-binding loop in EF5-hand motif of D. rerio secretagogin. A final 2mFo-DFc electron density map (blue mesh) is contoured at 1.2σ level. The refined protein model is shown in sticks. Residues of the calcium-binding motif at positions 1 (Asp206), 3 (Ser208), 5 (Thr210), 7 (Ala212) and 12 (Glu217) are labeled for clarity. A symmetry molecule of secretagogin from the crystalline lattice contributes Lys138 (cyan sticks), which mimics the positively charged calcium ion and stabilizes the loop in “Ca2+-ready”-like conformation.