Figure 2.
Effect of Tenovin-1 on BL2 and ARN8 Tumor Cell Lines and on Normal Human Dermal Fibroblasts
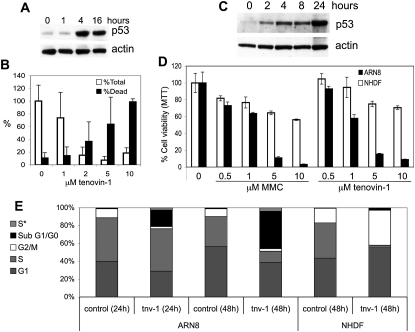
(A) BL2 Burkitt lymphoma cells were treated with 10 μM tenovin-1 for the indicated times, and p53 and actin were detected with DO1 or antibody CP01 (Calbiochem), respectively.
(B) BL2 cells were treated with increasing concentrations of tenovin-1 for 2 hr, after which tenovin-1 was removed and cells were cultured for 4 days in fresh medium. Cells were stained with trypan blue and counted. White bars represent the total number of cells in each sample (viable and nonviable) expressed as a percentage of the average number of cells in the untreated control samples. Black bars represent the percentage of dead cells in each sample (trypan blue-stained cells). Values correspond to the average of four independent experiments ± SD.
(C) ARN8 cells were treated with 10 μM tenovin-1 for the indicated times. p53 and actin were detected.
(D) Subconfluent ARN8 tumor cells or normal human dermal fibroblasts (NHDF) were treated with the indicated amounts of mitomycin C or tenovin-1 for 48 hr. Cell growth was determined by MTT assay (Smart et al., 1999). Values correspond to the average of three independent experiments ± SD.
(E) ARN8 tumor cells or normal human dermal fibroblasts (NHDF) were left untreated or treated with 10 μM tenovin-1 for the indicated times. Cell-cycle distribution was analyzed by BrdU labeling and FACS. S∗ indicates cells that do not incorporate BrdU and have a DNA content between 2N and 4N. Note that the proportion of cells with a sub-G1/G0 DNA content dramatically increases in the tenovin-1-treated ARN8 tumor cells. Instead, the effect of tenovin-1 on NHDFs is primarily cytostatic with little increase in the proportion of dead cells.