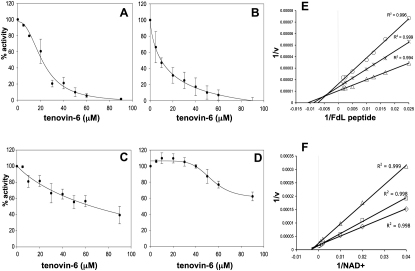
Figure 5.
Tenovin-6 Inhibits the Protein Deacetylase Activities of Purified Sirtuins SirT1 and SirT2
(A–D) Increasing concentrations of tenovin-6 were added to purified human SirT1 (A), SirT2 (B), SirT3 (C), or HDAC8 (D) reaction mixtures. Values correspond to the average enzyme activity of three independent experiments ± SD. Estimated IC50 values for SirT1, SirT2, and SirT3 in the assay conditions are 21, 10, and 67 μM, respectively.
(E) Analysis of tenovin-6's ability to compete for binding sites with the SirT1 FdL acetylated peptide substrate. In vitro SirT1 inhibition assay was carried out with tenovin-6 at 0, 50, and 75 μM with varying FdL concentrations and a constant NAD+ concentration of 1 mM. All assays contained the same amount of DMSO (0.25%). The data is presented as a Lineweaver-Burke plot (where the x axis intercept is −1/Km and the y axis intercept is 1/Vmax). Trend lines were then added to create a straight line. The trend lines for 0 μM (triangles), 50 μM (asterisks), and 75 μM (circles) tenovin-6 all have an R2 values above 0.99. Data points are the average of triplicate experiments.
(F) Analysis of tenovin-6's ability to compete for binding sites with SirT1 cosubstrate NAD+. In vitro SirT1 inhibition assay was carried out with tenovin-6 at 0, 25, and 50 μM with varying NAD+ concentrations and a constant FdL acetylated peptide concentration of 200 μM. All assays contained the same amount of DMSO (0.25%). The data are presented as a Lineweaver-Burke plot. The trendlines for 0 μM (diamonds), 25 μM (squares), and 50 μM (triangles) tenovin-6 all have an R2 value above 0.99. Data points are the average of triplicate experiments.