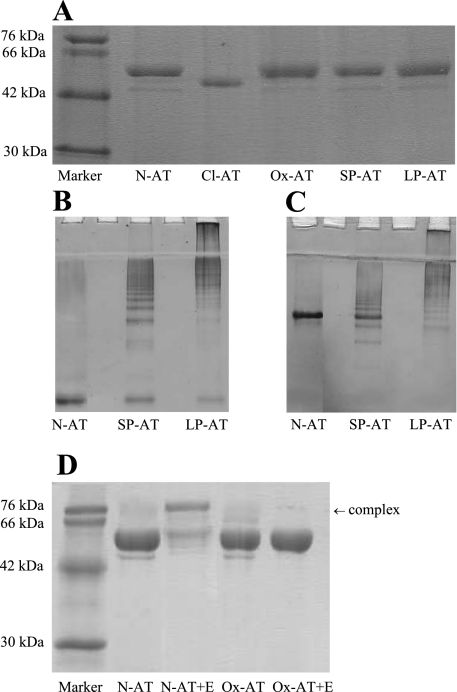
Fig. 1.
Characterization of conformations of α1-antitrypsin (AT). A: 12% (wt/vol) SDS-PAGE. Two micrograms of native AT (N-AT), Staphylococcus aureus V8 protease reactive loop-cleaved AT (Cl-AT), oxidized AT (Ox-AT), short-chain polymers (SP-AT), and long-chain AT polymers (LP-AT), were loaded onto 12% SDS-PAGE, 7.5% (wt/vol) nondenaturing PAGE with (B) and without (C) 8 M urea. Gel demonstrates difference in short- and long-chain polymers. Five micrograms of SP-AT and LP-AT were loaded per lane. Note the small amount of contamination of N-AT in SP-AT. D: 12% (wt/vol) SDS-PAGE demonstrating the difference in N-AT and Ox-AT. N-AT could form an SDS-stable complex with human neutrophil elastase, unlike Ox-AT.