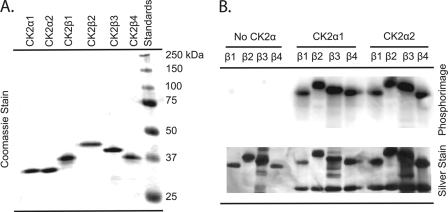
FIGURE 2.
SDS-PAGE analysis and autophosphorylation of A. thaliana CK2 subunits. A, purified recombinant A. thaliana CK2 subunits were separated by SDS-PAGE on a 12.5% gel and stained with Coomassie Brilliant Blue. Each lane contains 1.5 μg of the following: lane 1, CK2α1; lane 2, CK2α2; lane 3, CK2β1; lane 4, CK2β2; lane 5, CK2β3; lane 6, CK2β4; lane 7, molecular weight standards (Bio-Rad) are as indicated. B, reactions (100 μl) contained 50 pmol of β-subunit (CK2β1, CK2β2, CK2β3, or CK2β4) and were incubated in the presence or absence of either CK2α1 or CK2α2 (50 pmol) and [γ-32P]ATP (∼100–250 cpm/pmol). Reactions were separated by SDS-PAGE (12.5% gel) as described under “Experimental Procedures.” The gel was silver-stained, dried, and analyzed by phosphorimaging. Each lane contains ∼5 pmol of holoenzyme complex (10 μl of the reaction mixture). Lane 1, CK2β1; lane 2, CK2β2; lane 3, CK2β3; lane 4, CK2β4; lane 5, CK2α1β1; lane 6, CK2α1β2; lane 7, CK2α1β3; lane 8, CK2α1β4; lane 9, CK2α2β1; lane 10, CK2α2β2; lane 11, CK2α2β3; lane 12, CK2α2β4.