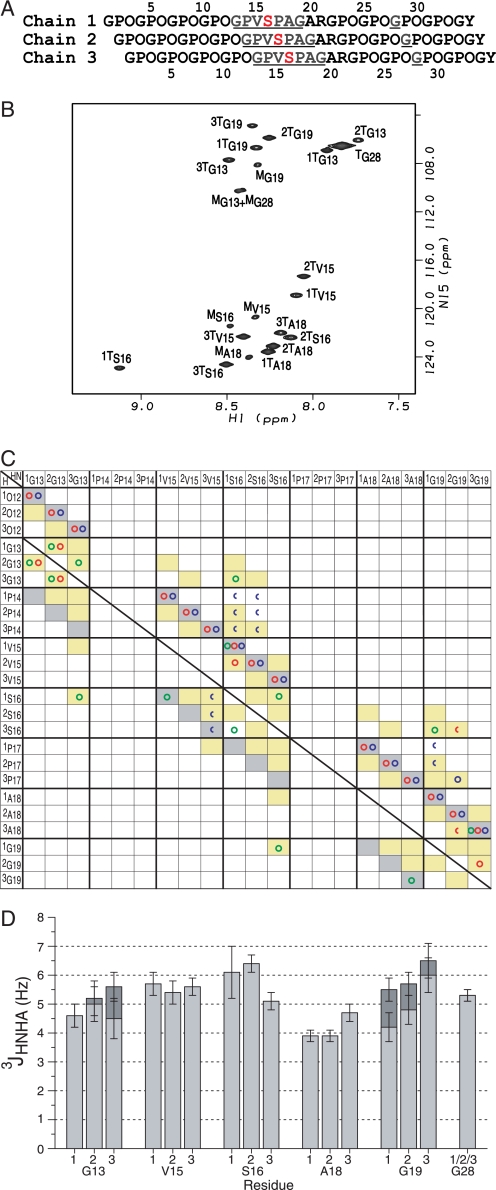
FIGURE 1.
NMR conformational characterization of the T1–898(G901S) peptide. A, the sequence diagram of the peptide shows the characteristic 1-residue stagger. Leading, middle, or trailing chain stagger assignment is indicated as chain 1, 2, or 3, respectively. The 13C/15N doubly labeled residues are underlined and colored in blue, and the Gly to Ser substation site is shown in red. B, the HSQC spectrum of the T1–898(G901S) peptide at 15 °C. The peaks corresponding to the monomer and trimer state are denoted with a superscript M or T, respectively. Chain stagger is indicated as a number in front of the superscript T. C, comparison of experimental NOEs of T1–898(G901S) peptide with a standard triple helical conformation. A predicted contact map obtained from a standard triple helix model structure is shown in shaded boxes as background (14). Contacts are shaded in gray for intrachain distances less than 5 Å and in yellow for interchain distances less than 5 Å. Experimental NOEs for T1–898(G901S) peptide are represented by circles (HN-HN (green circles), HN-Hα (red circles), and HN side chain protons (blue circles)) and are overlaid on the shaded contacts, showing the expected intrachain and interchain NOEs and one new contact (1Ser16 NH to 3Ser16 NH) consistent with the 1-residue stagger between chains and the packing of the Ser residues at the substitution site. Half circles represent overlapped NOEs. The superimposition of the experimental NOEs and the background indicates the 1-residue stagger of the triple helix throughout the substitution site. The diagnostic interchain NH–NH and NH–Hα NOEs between the three chains of the Gly13 residues (1Gly13NH to 2Gly13NH and 2Gly13Hα, 2Gly13NH to 1Gly13NH and 1Gly13Hα, 2Gly13NH to 3Gly13NH and 3Gly13Hα, 3Gly13NH to 2Gly13NH) and between Gly19 residues (3Gly19NH to 2Gly19Hα) are seen as well as interchain NOEs from 2Gly19NH to 3Pro17Hβ/γ supporting the 1-residue stagger. In addition, the NOEs normally unique between Gly are observed between the Ser16 residues and residues Gly13 and Gly19 (1Ser16NH to 3Gly13NH, 3Ser16NH to 1Gly19NH), suggesting that the Ser16 residues are packed into the center of the triple helix at the substitution site, in a behavior similar to a Gly at the same position. D, the histogram of the 3JHNHα coupling constants for the T1–898(G901S) peptide. 3JHNHα coupling constants from the two Hα Gly residues are shown in dark gray and light gray bars.