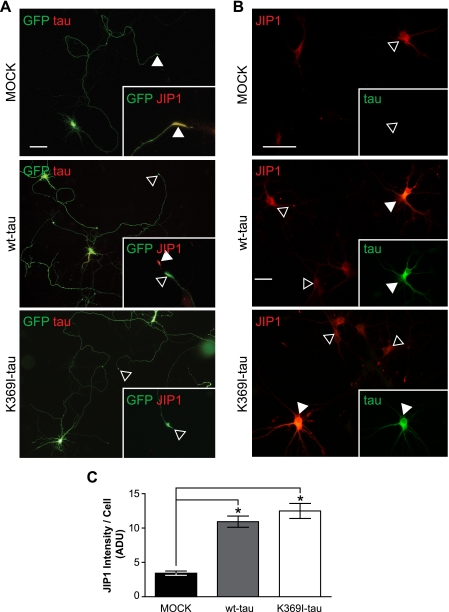
FIGURE 4.
Impaired axonal distribution of JIP1 is not caused by the K369I mutation of Tau. A, to determine whether the impaired axonal localization of JIP1 is due to the presence of K369I mutant Tau rather than elevated levels of hyperphosphorylated Tau per se, primary hippocampal neurons were transfected with MOCK, V5-wt-Tau, or V5-K369I mutant Tau together with green fluorescent protein (GFP) to visualize axonal tracing. In MOCK co-transfected neurons, JIP1 undergoes axonal transport and accumulates in growth cones (arrowheads; inset). In contrast, neurons co-transfected with V5-Tau (yellow merge) fail to accumulate JIP1 in growth cones (open arrowheads; inset). Note the intense JIP1 staining of the growth cone in untransfected neurons (arrowhead). Co-transfection of V5-K369I mutant Tau also results in a decreased JIP1 staining of growth cones. Scale bar, 50 μm. wt, wild type. B, co-transfection of primary hippocampal neurons with either V5-wt-Tau or V5-K369I mutant Tau together with Myc-JIP1 results in intensive JIP1 staining of the cell body (arrowheads), that is not found when the neurons are only transfected with the Myc-JIP1 construct (open arrowhead). Inset, staining for Tau. Scale bar, 50 μm. C, when staining is quantified, Myc-JIP1 fluorescence intensity shows increased levels of JIP1 in neurons that co-express either V5-wt-Tau or V5-K369I mutant Tau compared with co-transfection with an empty plasmid (MOCK; *, p < 0.0001). Thus, an increase of Tau in the cell body of neurons results in retention of JIP1, along with a decreased axonal distribution.