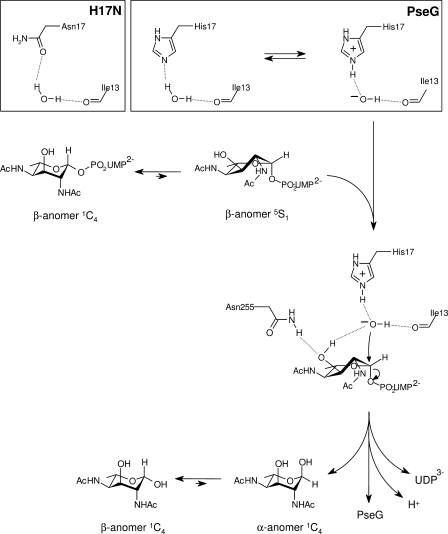
FIGURE 5.
Proposed catalytic mechanism for PseG based on modeling of the UDP-sugar complex and activity analysis of substitution derivatives. Here, PseG employs a single displacement mechanism involving C–O bond cleavage via direct attack of the anomeric carbon by a hydroxide nucleophile. Specifically, the residue His17 performs a base-catalyzed attack of water by abstracting a proton. The activated water is anchored with its other proton hydrogen-bonded to the main-chain carbonyl of Ile13, and it is further stabilized by the partially positively charged edge of the aromatic ring of Tyr78 (not shown). Several PseG residues participate in direct intermolecular interactions with the sugar moiety and stabilize the Michaelis complex, including Asn255 that appears as a determinant in selecting the twist-boat conformation already present in the free substrate. This twist-boat conformation of the sugar appears primed for catalysis, as it exposes the C-1 atom and favors attack by the His17-activated water, resulting in inversion of stereochemistry at C-1 and cleavage of the C-1–OGly bond. The liberated sugar product is expected to attain an equilibrium distribution of α and β anomers that interconvert nonenzymatically in solution. The inset shows a proposed scheme for His17 acting as a general base, abstracting a proton from water, or via the H17N substitution H-bonding with the proposed catalytic water molecule.