Table 1.
Synthesis of Seven- and Eight-Membered Ring Sultams via Intramolecular Oxa-Michael Reactions
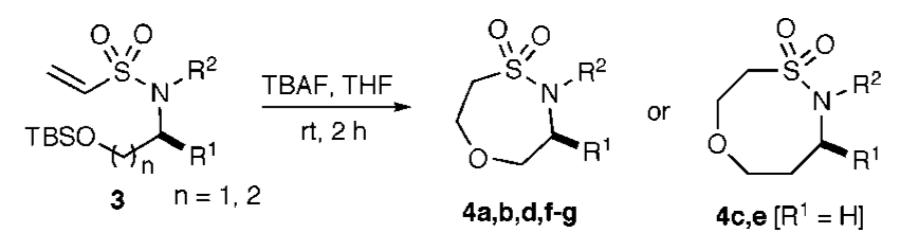 | |||||
|---|---|---|---|---|---|
| entry | product | n | R1 | R2 | total yielda (%) |
| 1 | 4a | 1 | i-Pr | CH2CH=CH2 | 61 |
| 2 | 4b | 1 | CH2Ph | CH2Ph(o-Br) | 60 |
| 3 | 4c | 2 | H | CH2CH=CH2 | 54 |
| 4 | 4d | 1 | CH2Ph | CH2Ph(o-Me) | 59b |
| 5 | 4e | 2 | H | CH2Ph(o-F) | 53b |
| 6 | 4f | 1 | i-Bu | CH2Ph(m-Cl) | 58b |
| 7 | 4g | 1 | i-Bu | CH2Ph | 61b |
Total yield of all four steps.
Sultams 4d—g were synthesized through chromatography-free linear synthesis; see Supporting Information for reaction procedure.
