Figure 2.
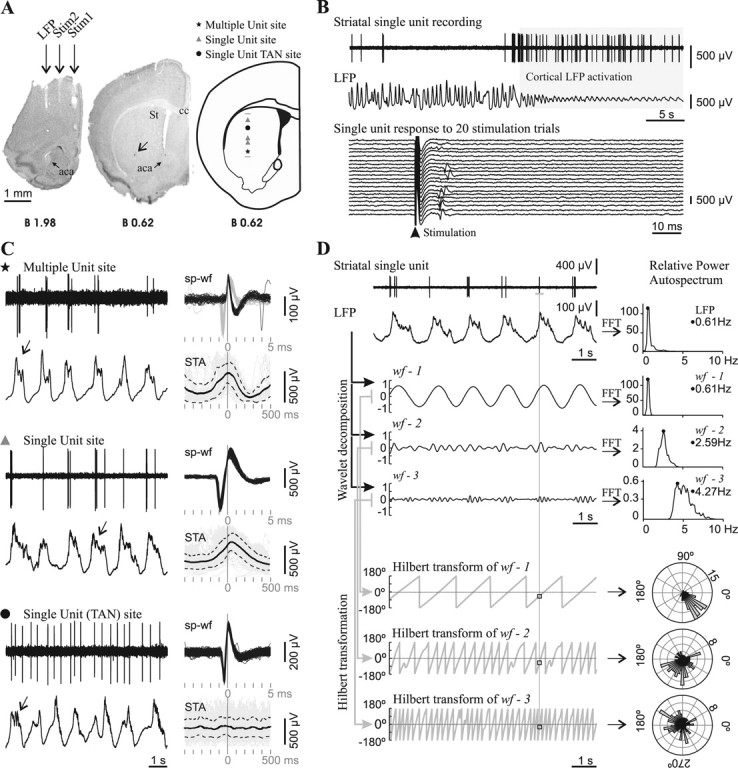
Spontaneous striatal spikes were phase locked to cortical field potential rhythms in adult control mice. A, Striatal neuronal discharge activity was recorded simultaneously with the motor cortex LFP in urethane-anesthetized mice. Photographs of coronal brain sections illustrate the position of electrodes in a representative experiment conducted in an adult mouse. The LFP electrode was positioned in the motor cortex area 1 [2 mm anterior to bregma, 1.5 mm lateral to midline, and 1.3 mm below cortical surface (Franklin and Paxinos, 2001)]. Two other concentric bipolar electrodes allowed electrical stimulation of the prefrontal cortex (Stim1: 2 mm anterior to bregma, 0.4 mm lateral, and 2.1 mm below cortical surface) and motor cortex area 2 (Stim2: 2 mm anterior to bregma, 1 mm lateral, and 1.3 mm below cortical surface). The arrow points to a Chicago Sky Blue deposit indicating the end of a glass microelectrode track in the striatum (St). Right, Schematic diagram of a coronal brain section (Franklin and Paxinos, 2001) illustrating the localization of all spontaneously active “single-unit” and “multiple-unit” type recording sites along a glass microelectrode track. aca, Anterior commissure, anterior; St, striatum; cc, corpus callosum. Scale bar, 1 mm. B, Mice striatal single units were modulated by the frontal cortex LFP and showed short latency excitatory responses to frontal cortex electrical stimulation. Top, The spontaneous activity of most striatal single units was modulated by slow-wave activity in adult mice. This striatal neuron fired exclusively during the positive part of the slow waves and showed increased spontaneous discharge during LFP activation. Bottom, The same unit fired a single spike with a short latency (10–20 ms) in response to electrical stimuli delivered to the motor cortex, in 35% of the trials at a stimulation current of 300 μA. C, Representative glass microelectrode recordings (left) taken from three sites along the track depicted in A, together with all spike waveforms (sp-wf) collected during a 3-min-long recording and the spike-triggered average (STA: solid black trace; SD: dotted traces) of LFP (right). Note that, in the two upper recordings, spikes occurred during the positive part of the slow wave, while in the recording site depicted at the bottom, spikes occurred throughout the slow wave. The presence of delta waves and spindles (arrows) on the top of the wave is an index of neuronal activity and indicates which is the “active part” of the slow wave (Kasanetz et al., 2006). The symbols indicate the position of the recording site along the track in A. TAN, Tonically active neuron. D, To assess phase locking of single-unit discharges to LFP oscillations (raw signals at upper left), the LFP was decomposed in three band-passed waveforms (wf-1, wf-2, wf-3) retaining different LFP frequencies, which were characterized by performing fast Fourier transforms (FFT) to compute relative power auto-spectra with 0.11 Hz resolution (right). wf-1 retained most of the power of the dominant slow-wave oscillation (range 0.4–1.5), wf-2 mainly reflected delta waves (1.5–4 Hz), and wf-3 retained frequency components related to spindles (4–8 Hz). The whole frequency range studied (0.4–10 Hz) encompassed >90% of the relative LFP spectral power when slow-wave activity was dominant. A Hilbert transformation (2 ms resolution) was performed to obtain the phase angle (gray sawtooth traces at bottom left) at every point of wf-1, wf-2, and wf-3, and to establish the instantaneous phase at the time of occurrence of every spike along the spike train (squares). Next, the number of spikes (radial axis) occurring at different phase angles (circular axis) of wf-1, wf-2, and wf-3 was depicted in circular plots (right; bin size: 7.2°). Phase locking of spike discharges is indicated by assessing deviation from uniformity and circular dispersion (see Materials and Methods). The illustrated neuron showed significant phase locking to slow-wave, delta, and spindle-related LFP activity.
