Abstract
Fenretinide (4HPR), a nontoxic analog of ATRA, has been investigated in various malignancies but not in multiple myeloma (MM), a plasma cell malignancy associated with induction of osteolytic bone disease. Here we show that 4HPR induces apoptosis through increased level of ROS and activation of caspase-8, 9 and 3, and inhibits growth of several MM cell lines in a dose-dependent manner. Serum or co-culture with the supportive osteoclasts partially protects MM cells from 4HPR-induced growth inhibition. Sphingosine-1 phosphate (S1P) significantly protects MM cells from 4HPR-induced apoptosis suggesting that as in other malignancies, this drug up-regulates ceramide in MM cells. 4HPR has no toxic effects on non-malignant cells such as blood mononucleated cells, mesenchymal stem cells and osteoblasts, but markedly reduces viability of endothelial cells and mature osteoclasts and inhibits differentiation of osteoclasts and MM-induced tube formation. 4HPR is a potential anti-MM agent, affecting MM cells and MM-induced bone disease and angiogenesis.
Keywords: fenretinide, multiple myeloma, osteoclastogenesis, sphingosine-1 phosphate, angiogenesis
1. Introduction
Multiple myeloma (MM) is characterized by the clonal expansion of monoclonal immunoglobulin-secreting plasma cells within the bone marrow (BM). Myeloma cells are often dependent on the BM microenvironment for growth and their dissemination within the hematopoietic BM are typically associated with induction of severe osteolytic bone disease [1] and angiogenesis [2]. Treatment with high dose therapy and novel agents substantially improved clinical outcome but treatment with these agents often associated with severe adverse effects and development of drug resistance [3]. These clinical observations emphasize the need of additional potent but yet relatively safe, anti-myeloma agents.
Fenretinide (N-(4-hydroxyphenyl) retinamide, or 4HPR), a neoclassical analog of the retinoids all-trans retinoid acid (ATRA), has been successfully tested as a chemopreventive and chemotherapeutic agent on various malignancies, such as prostate, breast and colorectal cancer [4]. It is less toxic and teratogenic than other retinoids [5], thereby making it one of the most promising retinoid anti-tumor compounds. Compared with ATRA, 4HPR exhibits reduced hepatotoxicity and increased efficacy in inhibiting mammary carcinogenesis in animal models [6]. Furthermore, 4HPR lacks the ability to induce point mutations or chromosomal aberrations, and is therefore not genotoxic [7]. 4HPR is currently under clinical evaluation in different cancers but the biological effect and therapeutic value in MM have not been investigated.
The cellular and molecular mechanisms by which 4HPR elicit its cytotoxic action are still not clearly understood. Compared with retinoic acid, a key distinction is that 4HPR induces cell apoptosis rather than differentiation and shows synergistic responses with chemotherapeutic drugs. Induction of apoptosis by 4HPR is mediated through a receptor-independent mechanism and is accompanied by increased cellular levels of ceramide[8,9] and ROS[10], and activation of caspases [11]. We reasoned that if ceramide is a major mediator of 4HPR-induced apoptosis, the effect of the drug could be counteracted by sphingosine-1-phosphate (S1P), which is another sphingolipid metabolite known to regulate cell growth and survival [12]. Whereas increased ceramide and sphingosine are associated with growth arrest and apoptosis, increased intracellular level of S1P is associated with suppression of apoptosis. Intracellularly, the balance between S1P and ceramide/sphingosine levels, which is regulated by a network of key enzymes, determines the fate of normal and malignant cells, and chemoresistance [12,13].
In this study, we have tested the effect of 4HPR on growth and survival of MM cells in the absence and presence the protective osteoclasts [14]. We also tested the effect of 4HPR on angiogenesis and osteoclastogenesis, two physiological processes typically stimulated in myelomatous bones.
2. Materials and methods
2.1. Reagents and supplies
An antibiotic mixture containing penicillin, streptomycin, and neomycin; α-minimum essential medium (α-MEM); and low-glucose Dulbecco’s modified Eagle’s medium (DMEM-LG) were obtained from Gibco (Grand Island, NY, USA). RPMI 1640 (without L-glutamine), Cellgro COMPLETE™ medium, were purchased from Mediatech, Inc (Herndon, VA, USA). Fetal bovine serum (FBS) was purchased from Hyclone (Logan, UT, USA). Recombinant human macrophage colony-stimulating factor (M-CSF) and RANKL were purchased from RDI (Flanders, NJ, USA). 4HPR was obtained from Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute. S1P was from Avanti Polar lipids, Inc (Alabaster, AL, USA).
2.2. Preparation of osteoclasts and osteoblasts
Cultures of multinucleated bone-resorbing osteoclasts were prepared as previously described [14]. Signed Institutional Review Board–approved informed consent forms were kept on record. The cells were cultured at 2.5 × 106/mL in α-MEM supplemented with 10% FBS, antibiotics, RANKL (50 ng/mL), and M-CSF (25 ng/mL) for 10 to 14 days, at which time they contained large numbers of multinucleatedosteoclasts with bone-resorbing activity.
Osteoblasts were prepared as previously described [15]. Briefly, mesenchymal stem cells (MSCs) were cultured in DMEM-LG medium supplemented with 10% FBS, dexamethasone (100 nM), β-glycerophosphate (10 mM), and ascorbate (0.05 mM) (osteoblastic medium) for approximately 3 weeks.
2.3. MM cells lines and osteoclast co-cultures
Two stromal cell dependent cell lines (BN and JB)[16] and two stromal independent MM cells lines (ARP-I, CAG) were maintained in our laboratory. MM cells were cultured in RPMI 1640 supplemented with 10% heat-inactivated FBS and antibiotics. Osteoclasts were washed three times with PBS to detach and remove non-adherent cells. MM cells were co-cultured with osteoclasts in RPMI 1640 medium with 10% FBS. At the end of each experiment, MM cells were collected and processed as previously reported [14] for further assays. At the end of each experiment MM cell growth was determined by MTT assay [17].
2.4. Terminal deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling (TUNEL)
MM cells were collected, cytospin slides prepared (40,000 cells/slide) and fixed in 10% phosphate-buffered formalin for 20 minutes in freshly prepared 4% paraformaldehyde (PFA). The TUNEL assay was performed using the Klenow FragEL™ DNA Fragmentation Detection Kit (Calbiochem, Darmstadt, Germany) according to the manufacturer’s instruction.
2.5. Annexin V/propidium iodide (PI) staining
MM cells treated for 24 hr with indicated concentrations of 4HPR were analyzed for apoptosis using Annexin V propidium iodide (PI) detection kit (Caltag Laboratories, Burlingame, CA, USA) by FacScan flow-cytometer (Becton Dickinson, San Jose, CA). Results were expressed as percent apoptotic annexin V+ cells. Adherent HUVEC cultured in 96-well plates were similarly stained for annexin V and total number of apoptotic annexin V+ cells counted using ZEISS AX10 Observer A1 microscope (Delta Optical Instruments, Thornwood, NY).
2.6. Western Blotting
Cytosolic and nuclear fractions were isolated with the use of the Nuclear/Cytosol Fractionation Kit (BioVision Research Products, Mountain View, CA). Equal amounts of lysate were separated by electrophoresis on 4% to 12% sodium dodecyl sulfate–polyacrylamide gels (Bio-Rad, Hercules, CA, USA), and Western blotting was carried out according to the Western Breeze chemiluminescent immunodetection protocol as described by the manufacturer (invitrogen; Carlsbad, California). Antibodies for caspase-3, 8, 9 (cell signaling technology; Danvers, MA, USA) were used.
2.7. Measurement of intracellular reactive oxygen species (ROS)
Production of ROS was detected using a 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) fluorescent probe obtained from invitrogen (Carlsbad, California). This compound is a cell-permeant indicator for ROS that is nonfluorescent until the acetate groups are removed by intracellular esterases and oxidation occurs within the cell. After treatment with 4HPR, H2DCFDA (5 μM) was added into the medium with MM cells in 24-well plate (0.2 × 106/well) 20 min before the end of treatment. Centrifuge cells and replace medium with 0.5 ml PBS, then analyze cells by flow cytometry (Becton Dickinson, San Jose, CA).
2.8. Matrigel Tube Formation Assay
BD Matrigel growth factor–reduced basement membrane matrix (Becton Dickinson, Franklin Lakes, NJ) was diluted on ice with DMEM medium (1:2 dilution factor), poured onto 96-well plates (100 μl/well), and incubated at 37°C for 30 minutes. Human umbilical vein endothelial cells (HUVEC; American Type Culture Collection, Manassas, VA) were cultured in Clonetics EBM-2 medium (Lonza Walkersville Inc., Walkersville, MD) supplemented with a cocktail of growth factors according the manufacturer’s instructions. For tube formation assay, HUVEC were trypsinized and seeded on Matrigel-containing chamber slides (15,000 cells/well) with indicated medium (100 μl/well) in the absence or the presence of 4HPR (1–10 μM) for 3 4 hours. Conditioned medium collected from a 48-hour culture of ARP-I or CAG MM cell lines (1.5 × 106 cells/mL) was used to test the effect of MM cells on tube formation. Tube-like structures per well were counted in triplicate with the use of a phase-contrast microscope. Images were acquired with a SPOT 2 digital camera and processed with Adobe Photoshop version 10.
2.9. Statistical analysis
Unless indicated otherwise, all values are expressed as mean±SEM. Student’s unpaired t-test was used to test the effect of 4HPR on cell growth, apoptosis, and numbers of tube-like shapes and osteoclasts.
3. Results
3.1. Effects of 4HPR on growth of peripheral blood mononucleated cells (PBMCs), MSC, HUVEC, osteoblasts and osteoclasts, and growth of MM cells in the absence and presence of serum and osteoclasts
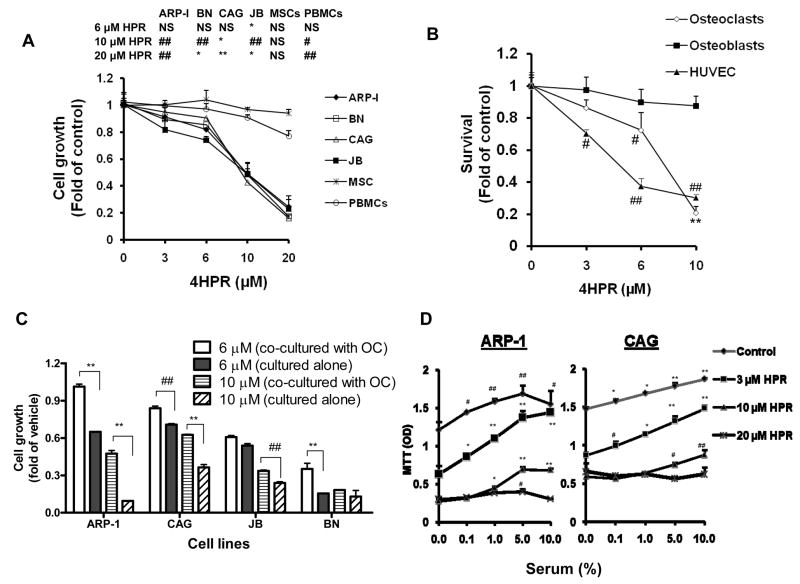
We initially tested the effect of 4HPR on survival and growth of MM cell lines and normal PBMCs and MSCs in the presence of 10% serum. 4HPR uniformly inhibited growth of ARP-1, BN, CAG, and JB MM cells in a dose related manner (0.5–20 μM, 48 hrs) as determined by MTT assay (Figure 1A). In contrast, similar concentrations of 4HPR had no significant toxicity on PBMCs or MSCs (Figure 1A). 4HPR also effectively inhibited growth of mature osteoclasts and HUVEC but had minimal toxic effect on osteoblasts’ survival (Figure 1B). We had demonstrated the stimulatory effects of osteoclastic cultures on MM cell survival [14]. Here we found that osteoclasts only partially, though significantly, protected MM cell lines from 4HPR-induced growth inhibition (Figure 1C).
Figure 1.
Effects of 4HPR on growth of non-malignant PBSCs, MSCs, HUVEC, osteoblasts and osteoclasts, and on MM cell growth in the absence and presence of serum and osteoclasts. A) MM cell lines (ARP-1, BN, CAG and JB), MSCs and PBMCs were treated with indicated concentrations of 4HPR in RPMI 1640 medium supplement with 10% serum for 48 hrs before subjected to MTT assay. Statistical analysis shown the table (upper panel) compared treatment groups versus control. B) Mature osteoclasts, osteoblasts and HUVEC were similarly treated with 4HPR in culture media for 48 hrs and assayed by MTT. C) MM Cells were co-cultured with osteoclasts in the presence or absence of 4HPR for 72 hrs in RPMI 1640 medium supplemented with 10% serum before subjected to MTT assay. D) MM cell lines (ARP-1 and CAG) were cultured in COMPLETE™ medium supplemented with different concentrations of serum and treated with indicated concentrations of 4HPR for 72 hrs. Statistical analysis shown compared Different serum concentrations versus control. Note that 4HPR was not toxic to PBMCs, MSCs or osteoblasts but inhibited growth of MM cell lines and mature osteoclasts in a dose-dependent manner. Serum and osteoclasts partially protected MM cells from 4HPR-induced growth inhibition. (# p<0.05; ## p<0.01; * p<0.001; ** p<0.0001)
Since many of 4HPR’s effects are mediated through increased intracellular ceramide [8, 9] and S1P counteracts ceramide actions, we sought to test the effect of increased concentration of serum, which contains S1P released from platelet after activation [18], on the 4HPR-induced MM cell-growth inhibition. We found that increased serum concentrations markedly protected MM cells from low dose of 4HPR (3 μM), and had partial or no protective effect at high concentrations of 4HPR (10–20 μM, Figure 1D); we therefore utilized serum free medium to study the effect of 4HPR and S1P on MM cell survival and growth.
3.2. 4HPR-induced MM cell apoptosis is counteracted by S1P
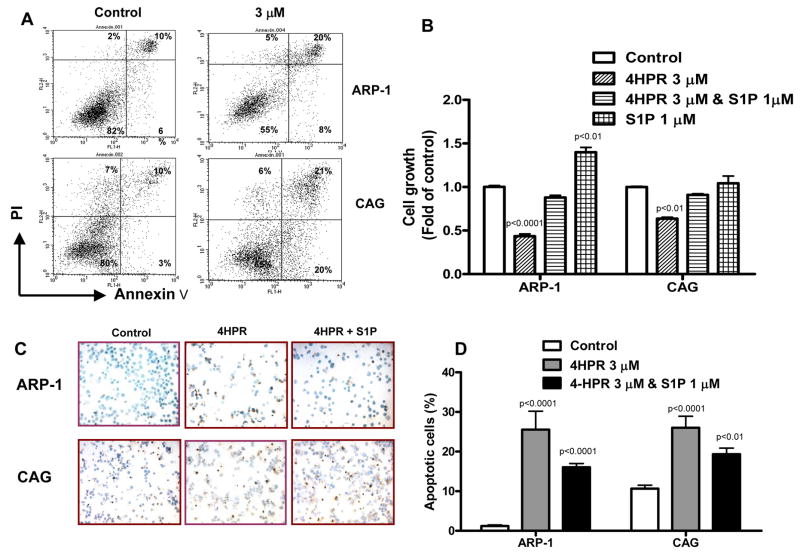
Using annexin V/PI flow analysis we found that 4HPR (3 μM) significantly increased the percent of annexin V+ (apoptotic) MM cells following 24 hrs of culture in serum-free conditions (Figure 2A). Next we tested the effect of S1P on 4HPR-induced MM cell growth inhibition. In COMPLETE™ media (serum-free conditions) S1P promoted growth of ARP1 but not CAG cells and protected the two cell lines from the inhibitory effect of 4HPR (Figure 2B). We failed to determine the effect of S1P on MM cell apoptosis by annexin V/PI flow cytometry, due to altered cell membrane properties by S1P [19]. Instead, we sought to analyze the effect of S1P on 4HPR-induced apoptosis using the TUNEL assay. As with annexin V/PI flow analysis, the TUNEL assay demonstrated induction of MM cell apoptosis by 4HPR. S1P partially but significantly protected MM cells from 4HPR-induced apoptosis (Figure 2C, D). Our data suggest that S1P is mainly a survival rather than growth factor for MM cell lines.
Figure 2.
4HPR induces apoptosis in MM cell lines, an effect that is partially attenuated by S1P. ARP-1 and CAG MM cell lines were cultured in serum-free COMPLETE™ medium and treated with indicated concentrations of 4HPR for indicated time. A) Annexin V/PI flow cytometry analysis. 24 hrs incubation with 4HPR increased percentage of annexin V+ (apoptotic) MM cells. B) ARP-1 and CAG cells were cultured in serum-free COMPLETE™ medium and in the absence and presence of 4HPR and/or S1P for 72 hours, and then subjected to MTT assay. S1P significantly attenuated the effect of 4HPR on MM cell growth. C, D) ARP-1 and CAG cells were cultured in serum-free COMPLETE™ medium and treated with 4HPR in presence and or absence of S1P for 24 hours and then subjected to TUNEL assay (original magnification ×100). Note that the percentage of TUNEL (apoptotic) positive cells (stained dark-brown in C) was increased by 4HPR, and that S1P significantly counteracted this effect (D).
3.3. The proapoptotic effect of 4HPR involves increased level of ROS and activation of caspase-8, 9 & 3
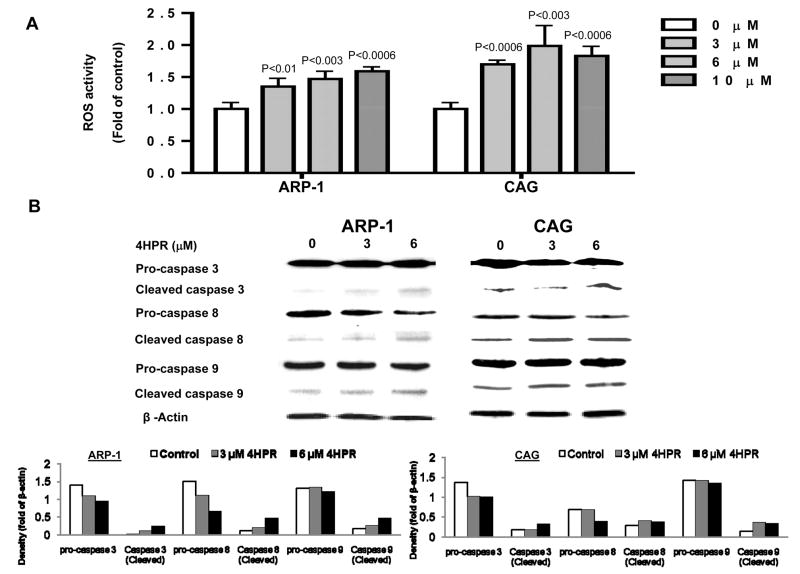
We measured ROS levels in ARP-I and CAG MM cells following two hours of treatment with 4HPR. 4HPR increased ROS levels in MM cells in a dose-dependent manner (Figure 3A). Examination of caspases activity by Western blotting following 24 hours of treatment with 4HPR revealed decreased levels of pro-caspase-3, 8 and 9, and increased levels of cleaved caspase-3, 8 and 9 in ARP-I and CAG MM lines (Figure 3B). These data suggest that the 4HPR-induced apoptosis of MM cells is mediated through death receptor and mitochondria pathways.
Figure 3.
4HPR increases ROS levels and activates caspase 3, 8 and 9 in MM cells. A) ARP-1 or CAG MM cells were treated with indicated concentrations of 4HPR for 2 hours before adding the H2DCFDA dye and analyzing levels of ROS by flow cytometry (see “Materials and Methods”). B) MM cell lines (ARP-I, CAG) were treated with 3 or 6 μM of 4HPR for 24 hrs and activation of caspase 3, 8, and 9 were detected by Western blot.
3.4. 4HPR suppresses MM-induced tube formation and osteoclastogenesis
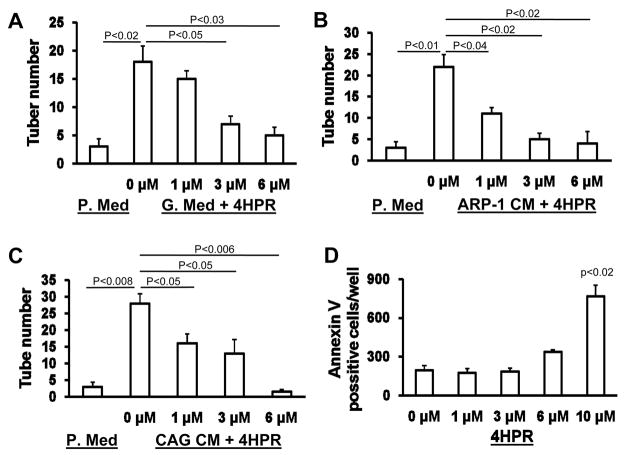
We investigated the effects of 4HPR on the MM-induced angiogenesis and human osteoclastogenesis which are two typical manifestations associated with myeloma cell growth in the BM [20, 21]. We initially confirmed the inhibitory effect of 4HPR on tube formation by HUVEC on Matrigel in media containing a cocktail of angiogenic growth factors (Figure 4A) [22]. Next we demonstrated that MM cell conditioned media stimulated tube formation by HUVEC and that this effect was significantly inhibited by 4HPR at 1 fÊM or higher concentrations (Figure 4B, C). To exclude the possibility that inhibition of tube formation was not a consequence of induction of apoptosis by 4HPR, we treated HUVEC with increasing concentrations of 4HPR for a similar period of time (4 hours). We found that at this conditions, 4HPR significantly increased number of apoptotic HUVEC at concentrations that exceed 6μM (Figure 4D). These results suggest that 4HPR inhibits tube formation and HUVECs’ survival.
Figure 4.
4HPR inhibits MM-induced tube formation by HUVECs. A-C) HUVEC (15,000 cells/well) were cultured on Matrigel in 96-well plates for 3 hrs in the presence or absence of indicated concentrations of 4HPR. Tube formations were tested in plain medium (P. Med), HUVEC growth medium (G. Med), ARP-I conditioned medium (ARP-1 CM, 50% in plain medium) and CAG cell conditioned medium (CAG CM, 50% in plain medium). Note that 4HPR inhibited tube formation induced by growth factors or MM cell conditioned media in a dose-depended manner. D) HUVEC were cultured in 96-well plate (10,000 cells per well) and treated with indicated concentrations of 4HPR for 4 hours. Following treatment HUVEC cells were washed with PBS and stained with Annexin V according to the manufacturer’s instructions (see Materials and Methods). Note that under the experimental conditions 4HPR induced apoptosis at concentrations higher than 6 μM.
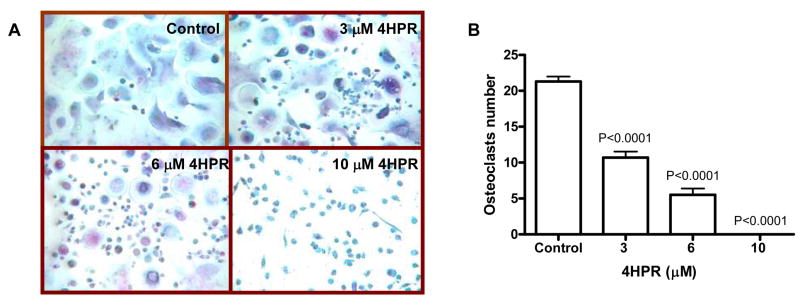
4HPR has been shown to inhibit osteoclastic differentiation of murine microphage-like cell line [23]. To test whether 4HPR also affects primary human osteoclastogenesis, human osteoclast precursors[14] were cultured in osteoclastic medium in the presence and absence of 4HPR for 7 days. During this time, many multinucleated osteoclasts formed in control cultures but the number of osteoclasts was dose-dependently suppressed by 4HPR (Figure 5A, B). These experiments suggest that 4HPR may also inhibit angiogenesis and osteoclastogenesis.
Figure 5.
4HPR inhibits differentiation of primary human osteoclast precursors. A, B) Human osteoclast precursors were cultured in osteoclastic growth media (see “Materials and Methods”) in the presence or absence of indicated concentrations of 4HPR for 7 days. Osteoclast differentiation was determined by counting the number of multinucleated TRAP-expressing osteoclasts. As shown in A, numerous osteoclasts were formed in cultures treated with vehicle (Control) but not 4HPR (original magnification ×100). 4HPR reduced number of osteoclasts in a dose-dependent manner.
4. Discussion
In the current study we show that 4HPR inhibits survival and growth of several MM cell lines and that these effects are partially attenuated by coculture with the supportive osteoclasts[14]. In contrast, 4HPR has minimal toxic effect on non-malignant cells (e.g. PBMCs, MSC, osteoblasts). 4HPR has been previously shown to inhibit angiogenesis [22] and differentiation of murine microphage--like cell line [23]. For the first time we showed the ability of 4HPR to suppress MM cell–induced tube formation by HUVECs. Using primary human osteoclasts, we demonstrate that in addition to its inhibitory effect on osteoclast differentiation, 4HPR also reduces survival of mature, primary human osteoclasts. It has been suggested that the dynamic balance between intracellular S1P and ceramide, the “sphingostat”, and the consequent regulation of opposing signaling pathways, are important factors determining cell fate [24]. We here show that S1P moderately promote MM cell growth by itself, but serum and exogenous S1P counteract the inhibitory effect of 4HPR on MM cell growth, suggesting that, as previously reported [8, 9], ceramide is a major mediator of 4HPR action. In human plasma, S1P concentration range between 0.2 and 1 μM, and it is released into the blood stream mainly upon platelet activation [18, 25]. Interestingly, osteoclasts also have been show to produce S1P [26] and protect MM cells from spontaneous and drug-induced apoptosis [14,15]. Whether S1P mediates the protective effect of serum and osteoclasts from 4HPR-induced apoptosis is a matter of continual investigation. Overall, our study suggests that 4HPR is a potential agent for treating MM and its associated osteolytic bone disease.
The ability of 4HPR to inhibit MM cell growth in co-culture with osteoclasts could be mediated through direct effect of the drug on osteoclast viability and/or function. Of note, however, 4HPR inhibited growth of MM cells in co-culture with osteoclasts at a concentration (6 μM) that had minimal effect on osteoblast survival (Figure 1B). The inability of osteoclasts to completely protect MM cells could also be due to secretion of low level of S1P (and perhaps other similar sphingolipid members) which insufficiently protects MM cells from 4HPR-induced apoptosis.
4HPR elicits a transient rise in endogenous ceramide levels in various neoplastic cells [13]. Ceramide is a sphingolipid second messenger that is generally recognized to promote apoptosis in response to inflammatory cytokines like Fas ligand and TNF, as well as conditions associated with oxidative stress. Increasing ceramide activate the transcription factor of NF-κB, protein phosphatase kinase and elevation in ROS [27, 28]. The ability of 4HPR to increase ROS in MM cells and subsequent activation of caspase 3, 8 and 9 further suggest that 4HPR induces apoptosis in MM cells through both death receptor and mitochondria pathways as reported fo r other malignancies[29,30].
We conclude that 4HPR may represent a relatively safe and nontoxic anti-myeloma agent affecting survival of MM cells and reducing bone disease through inhibition of osteoclast differentiation and viability.
Acknowledgments
This work was supported by grants from the National Cancer Institute (CA-93897) (S.Y.) and from the Multiple Myeloma Research Foundation (Senior and Translational Research Awards) (S.Y.).
Footnotes
Authors have no conflict of interest to disclose.
References
- 1.Lentzsch S, Ehrlich LA, Roodman GD. Pathophysiology of multiple myeloma bone disease. Hematol Oncol Clin North Am. 2007;21:1035–1049. doi: 10.1016/j.hoc.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Vacca A, Ribatti D. Bone marrow angiogenesis in multiple myeloma. Leukemia. 2006;20:193–199. doi: 10.1038/sj.leu.2404067. [DOI] [PubMed] [Google Scholar]
- 3.Richardson PG, Mitsiades C, Schlossman R, Munshi N, Anderson K. New drugs for myeloma. Oncologist. 2007;12:664–689. doi: 10.1634/theoncologist.12-6-664. [DOI] [PubMed] [Google Scholar]
- 4.Decensi A, Costa A. Recent advances in cancer chemoprevention, with emphasis on breast and colorectal cancer. Eur J Cancer. 2000;36:694–709. doi: 10.1016/s0959-8049(00)00040-x. [DOI] [PubMed] [Google Scholar]
- 5.Lieberman R, Crowell JA, Hawk ET, Boone CW, Sigman CC, Kelloff GJ. Development of new cancer chemoprevention agents: role of pharmacokinetic/pharmacodynamic and intermediate endpoint biomarker monitoring. Clin Chem. 1998;44:420–427. [PubMed] [Google Scholar]
- 6.Ulukaya E, Wood EJ. Fenretinide and its relation to cancer. Cancer Treat Rev. 1999;25:229–235. doi: 10.1053/ctrv.1999.0127. [DOI] [PubMed] [Google Scholar]
- 7.Paulson JD, Oldham JW, Preston RF, Newman D. Lack of genotoxicity of the cancer chemopreventive agent N-(4-hydroxyphenyl)retinamide. Fundam Appl Toxicol. 1985;5:144–150. doi: 10.1016/0272-0590(85)90058-2. [DOI] [PubMed] [Google Scholar]
- 8.Rehman F, Shanmugasundaram P, Schrey MP. Fenretinide stimulates redox-sensitive ceramide production in breast cancer cells: potential role in drug-induced cytotoxicity. Br J Cancer. 2004;91:1821–1828. doi: 10.1038/sj.bjc.6602212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrews WJ, Winnett G, Rehman F, Shanmugasundaram P, Hagen D, Schrey MP. Aromatase inhibition by 15-deoxy-prostaglandin J(2) (15-dPGJ(2)) and N-(4-hydroxyphenyl)-retinamide (4HPR) is associated with enhanced ceramide production. J Steroid Biochem Mol Biol. 2005;94:159–165. doi: 10.1016/j.jsbmb.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Oridate N, Suzuki S, Higuchi M, Mitchell MF, Hong WK, Lotan R. Involvement of reactive oxygen species in N-(4-hydroxyphenyl)retinamide-induced apoptosis in cervical carcinoma cells. J Natl Cancer Inst. 1997;89:1191–1198. doi: 10.1093/jnci/89.16.1191. [DOI] [PubMed] [Google Scholar]
- 11.Wu JM, DiPietrantonio AM, Hsieh TC. Mechanism of fenretinide (4-HPR)-induced cell death. Apoptosis. 2001;6:377–388. doi: 10.1023/a:1011342220621. [DOI] [PubMed] [Google Scholar]
- 12.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 13.Hail N, Jr, Kim HJ, Lotan R. Mechanisms of fenretinide-induced apoptosis. Apoptosis. 2006;11:1677–1694. doi: 10.1007/s10495-006-9289-3. [DOI] [PubMed] [Google Scholar]
- 14.Yaccoby S, Wezeman MJ, Henderson A, Cottler-Fox M, Yi Q, Barlogie B, Epstein J. Cancer and the microenvironment: myeloma-osteoclast interactions as a model. Cancer Res. 2004;64:2016–2023. doi: 10.1158/0008-5472.can-03-1131. [DOI] [PubMed] [Google Scholar]
- 15.Yaccoby S. The phenotypic plasticity of myeloma plasma cells as expressed by dedifferentiation into an immature, resilient, and apoptosis-resistant phenotype. Clinical Cancer Res. 2005;11:7599–7606. doi: 10.1158/1078-0432.CCR-05-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Pennisi A, Zhan F, Sawyer J, Shaughnessy J, Yaccoby S. Establishment and exploitation of hyperdiploid and non-hyperdiploid human myeloma cell lines. Br J Haematol. 2007;138:802–811. doi: 10.1111/j.1365-2141.2007.06742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yaccoby S, Wezeman MJ, Zangari M, Walker R, Cottler-Fox M, Gaddy D, Ling W, Saha R, Barlogie B, Tricot G, Epstein J. Inhibitory effects of osteoblasts and increased bone formation on myeloma in novel culture systems and a myelomatous mouse model. Haematologica. 2006;91:192–199. [PMC free article] [PubMed] [Google Scholar]
- 18.Yatomi Y. Plasma sphingosine 1-phosphate metabolism and analysis. Biochim Biophys Acta. 2008;1780:606–611. doi: 10.1016/j.bbagen.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 19.van Engeland M, Nieland L, Ramaekers F, Schutte B, Reutelingsperger CP. Annexin V-Affinity assay: A review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31:1–9. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 20.Bataille R, Chappard D, Marcelli C, Dessauw P, Baldet P, Sany J, Alexandre C. Recruitment of new osteoblasts and osteoclasts is the earliest critical event in the pathogenesis of human multiple myeloma. J Clin Invest. 1991;88:62–66. doi: 10.1172/JCI115305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vacca A, Ribatti D, Roncali L, Ranieri G, Serio G, Silvestris F, Dammacco F. Bone marrow angiogenesis and progression in multiple myeloma. Br J Haematol. 1994;87:503–508. doi: 10.1111/j.1365-2141.1994.tb08304.x. [DOI] [PubMed] [Google Scholar]
- 22.Noonan DM, Benelli R, Albini A. Angiogenesis and cancer prevention: a vision. Recent Results Cancer Res. 2007;174:219–224. doi: 10.1007/978-3-540-37696-5_19. [DOI] [PubMed] [Google Scholar]
- 23.Shishodia S, Gutierrez AM, Lotan R, Aggarwal BB. N-(4-hydroxyphenyl)retinamide inhibits invasion, suppresses osteoclastogenesis, and potentiates apoptosis through down-regulation of I(kappa)B(alpha) kinase and nuclear factor-kappaB-regulated gene products. Cancer Res. 2005;65:9555–9565. doi: 10.1158/0008-5472.CAN-05-1585. [DOI] [PubMed] [Google Scholar]
- 24.Maceyka M, Milstien S, Spiegel S. Shooting the messenger: oxidative stress regulates sphingosine-1-phosphate. Circ Res. 2007;100:7–9. doi: 10.1161/01.RES.0000255895.19868.a3. [DOI] [PubMed] [Google Scholar]
- 25.Melendez AJ. Sphingosine kinase signalling in immune cells: potential as novel therapeutic targets. Biochim Biophys Acta. 2008;1784:66–75. doi: 10.1016/j.bbapap.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Ryu J, Kim HJ, Chang EJ, Huang H, Banno Y, Kim HH. Sphingosine 1-phosphate as a regulator of osteoclast differentiation and osteoclast-osteoblast coupling. EMBO J. 2006;25:5840–5851. doi: 10.1038/sj.emboj.7601430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dobrowsky RT, Kamibayashi C, Mumby MC, Hannun YA. Ceramide activates heterotrimeric protein phosphatase 2A. J Biol Chem. 1993;268:15523–15530. [PubMed] [Google Scholar]
- 28.Yang Z, Costanzo M, Golde DW, Kolesnick RN. Tumor necrosis factor activation of the sphingomyelin pathway signals nuclear factor kappa B translocation in intact HL-60 cells. J Biol Chem. 1993;267:20520–20523. [PubMed] [Google Scholar]
- 29.Sun SY, Li W, Yue P, Lippman SM, Hong WK, Lotan R. Mediation of N-(4-hydoxyphenyl)retinamide-induced apoptosis in human cancer cells by different mechanisms. Cancer Res. 1999;59:2493–2498. [PubMed] [Google Scholar]
- 30.Kim HJ, Chakravarti N, Oridate N, Choe C, Claret FX, Lotan R. N-(4-hydroxyphenyl)retinamide-induced apoptosis triggered by reactive oxygen species is mediated by activation of MAPKs in head and neck squamous carcinoma cells. Oncogene. 2006;25:2785–2794. doi: 10.1038/sj.onc.1209303. [DOI] [PMC free article] [PubMed] [Google Scholar]