Figure 3. High-affinity and specific interaction between of LRP4-neuronal agrin.
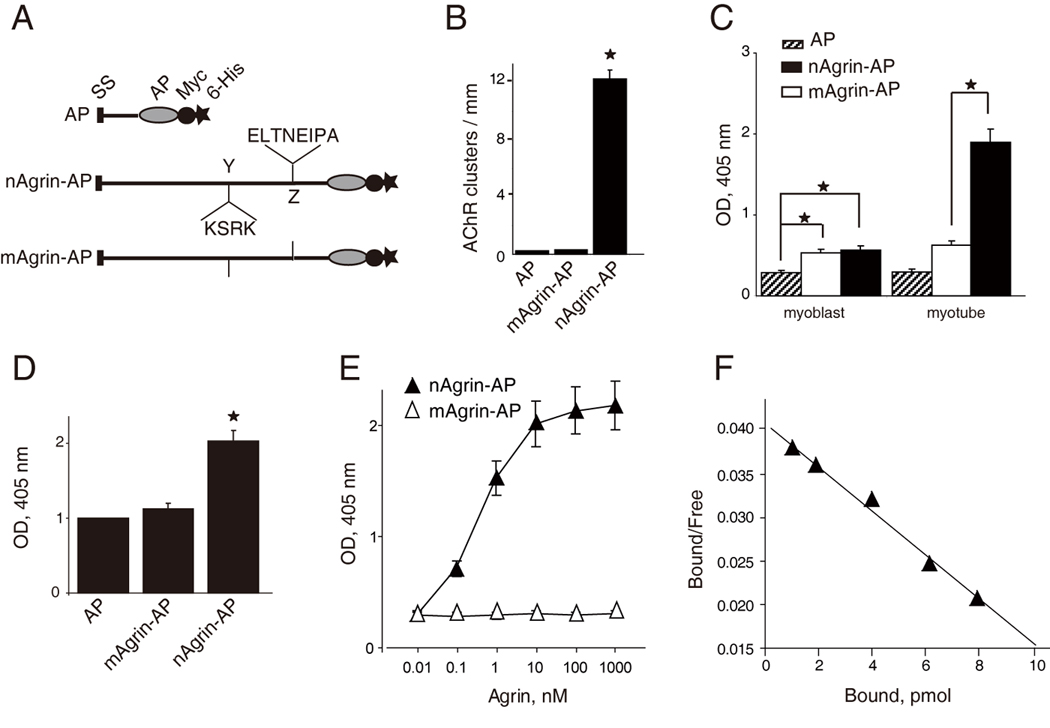
(A) Schematic diagrams of AP constructs. Neuronal or muscle agrin was fused to AP in pAPtag-5. The fusion proteins contain a signal peptide (SS) in the N-terminus, and two additional tags (Myc and His) in the C-terminus. Neuronal agrin contains 4- and 8-amino acid residue inserts at the Y and Z sites, respectively.
(B) Functional characterization of agrin-AP recombinant proteins. C2C12 myotubes were stimulated with AP alone, mAgrin-AP or nAgrin-AP for 18 hr. AChR clusters were assayed as described in Experimental Procedures. Data shown were mean ± SEM. n = 4; *, P < 0.05 in comparison with AP or mAgrin-AP.
(C) Differential binding activities of mAgrin-AP and nAgrin-AP to myoblasts and myotubes. C2C12 myoblasts and myotubes were incubated AP alone, mAgrin-AP or nAgrin-AP for 90 min at room temperature. Endogenous AP was inactivated by heating and bound AP was assayed by staining with BCIP/NBT. Data shown were mean ± SEM. n = 6; *, P < 0.05.
(D) Direct interaction between LRP4 and neuronal agrin. LRP4-Myc was purified and coated on Maxi-Sorp Immuno Plates, which were incubated with nAgrin-AP or mAgrin-AP. AP activity was measured with pNPP as substrate. Control, condition medium of HEK293 cells transfected with the empty pAPtag-5. Data shown were mean ± SEM. n = 3; *, P < 0.05 in comparison with AP or mAgrin-AP.
(E) Dose-dependent interaction between LRP4 and neuronal Agrin. Purified LRP4-Myc was coated on Maxi-Sorp Immuno Plates, which were incubated with nAgrin-AP or mAgrin-AP. AP activity was measured with pNPP as substrate. Data shown were mean ± SEM. n = 4; *, P < 0.05.
(F) Scatchard plot of data in E. Y axis represents the ratio of bound to free nAgrin-AP whereas X axis represents the concentration of bound nAgrin-AP.
