Table 1.
Reduction of Benzylic and Allylic Halides with Pyridine N-Oxide in CH3CN
| Entry | Substrate | Product | Temperature | Yield (isolated) |
|---|---|---|---|---|
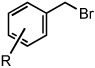 |
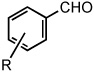 |
|||
| 1 | R = H | 25 °C | 82% | |
| 2 | R = p-Br | 25 °C | 80% | |
| 3 | R = p-CO2CH3 | 25 °C | 81% | |
| 4 | R = m-CN | 50 °C | 84% | |
| 5 | R = p-CN | 50 °C | 80% | |
| 6 | R = m-NO2 | 50 °C | 86% | |
| 7 | R = p-NO2 | 50 °C | 86% | |
| 8 | 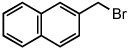 |
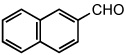 |
25 °C | |
| 9 | 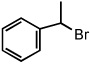 |
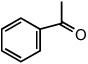 |
25 °C | 84% |
| 10 | 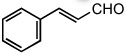 |
25 °C | 85% | |
| 11 | 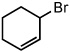 |
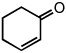 |
25 °C | 85% |
| 12 | 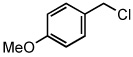 |
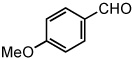 |
50 °C | 92% |
| 13 | 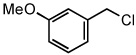 |
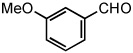 |
50 °C | 74%a |
| 14 | 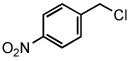 |
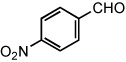 |
50 °C | 75%b |
| 15 | 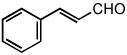 |
50 °C | 95% | |
The yield is based on 71% converted starting material.
The yield is based on 50% converted starting material.
