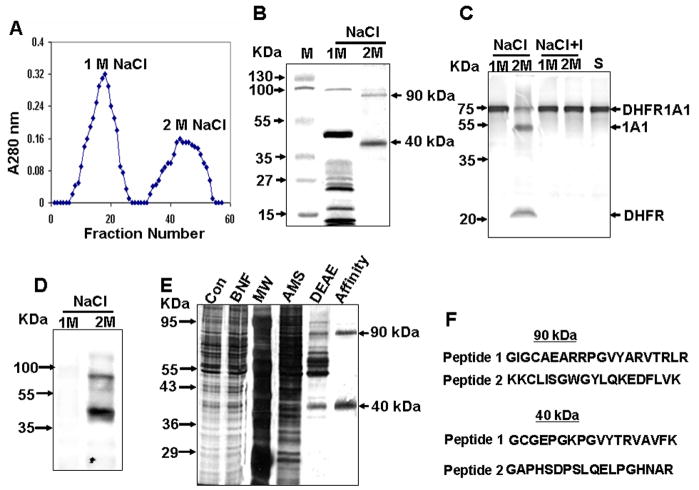
Figure 2. Endoprotease Purification by Aprotinin-Agarose Affinity Chromatography.
(A) Elution pattern of aprotinin-agarose column-bound proteins. The DEAE Sephacel-purified fraction (IV) was loaded onto the aprotinin-agarose column, and bound proteins were eluted using a NaCl step gradient.
(B) Cypro Ruby-stained protein fractions separated by SDS-PAGE.
(C) Proteolytic activity of protein fractions, as determined using 35S-labeled DHFR-1A1 as substrate. “S” indicates substrate alone, and “I” indicates reactions performed in the presence of Pefabloc.
(D) Far western blot analysis of fractions using aprotinin.
(E) Polyacrylamide gel electrophoresis (12%) of fractions at each progressive purification stage. Proteins shown, from left to right, are total liver cytosolic protein (Con, 10 μg protein), the liver cytosolic fraction from BNF-treated animals (BNF, 10 μg), molecular weight markers (MW), the 50% (NH4)2SO4 fraction (AMS, 10 μg), the peak IV DEAE sephacel-purified fraction (DEAE, 3 μg), and the aprotinin-agarose purified fraction (Affinity, 0.5 μg).
(F) Peptide sequences for p90 and p40.