Figure 9.
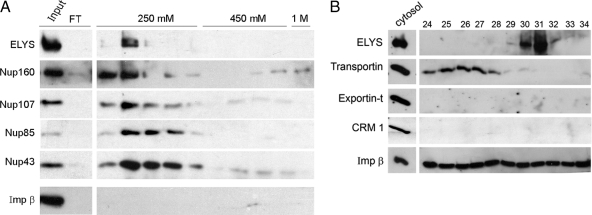
A RanGTP affinity column pulls out ELYS and the Nup107-160 complex from cytosol. (A) ELYS-enriched fractions eluted from a Q-Sepharose column were pooled and loaded on an affinity column of immobilized RanQ69L-GTP. Samples were analyzed by immunoblotting. Equivalent samples of the starting material and the flowthrough fraction of the RanQ69L-GTP column were loaded in the first two lanes. ELYS, Nup107-160, and importin β were retained on the affinity column. The column was washed with four column volumes and sequentially eluted with buffer containing 250 mM, 450 mM, and 1 M KCl. Samples from consecutive eluted fractions were loaded for each step. Only ELYS and Nup107-160 complex members were eluted in the first step, whereas importin β remained bound to the column. (B) Fractions eluted from a Q-Sepharose column were probed with antibodies directed against four different transport receptors of the importin β superfamily and compared with a sample of complete cytosol (0.3 μl). Importin β was present in all of these fractions, CRM 1 and exportin-t were not detected, whereas transportin peaked in fractions 24–28, showing minimal overlap with ELYS/Nup107-160.