Table 2.
Library Data for Compounds 7{1-12}
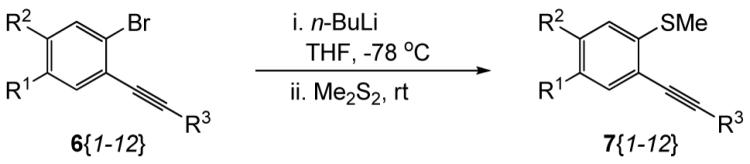 | ||||
|---|---|---|---|---|
| compd 7 | R1 | R2 | R3 | yield(%)a |
| 7{1} | MeO | H | 4-MeOC6H4 | 86 |
| 7{2} | MeO | H | 3-MeOC6H4 | 87 |
| 7{3} | MeO | H | 2-MeOC6H4 | 93 |
| 7{4} | MeO | H | 3,5-(MeO)2C6H3 | 63 |
| 7{5} | MeO | H | 4-Me2NC6H4 | 79 |
| 7{6} | H | MeO | 4-MeOC6H4 | 89 |
| 7{7} | H | MeO | 2-MeOC6H4 | 91 |
| 7{8} | MeO | MeO | 4-MeOC6H4 | 83 |
| 7{9} | MeO | MeO | 2-MeOC6H4 | 81 |
| 7{10} | MeO | MeO | 3-thiophenyl | 79b |
| 7{11} | 4-MeOC6H4 | 63 | ||
| 7{12} | 2-MeOC6H4 | 90 | ||
Isolated yields after column chromatography. All compounds 7 have been characterized by 1H and 13C NMR spectroscopy.
An inseparable mixture was obtained.
