Abstract
The neural mechanisms whereby placebo conditioning leads to placebo analgesia remain unclear. In this study we aimed to identify the brain structures activated during placebo conditioning and subsequent placebo analgesia. We induced placebo analgesia by associating a sham treatment with pain reduction and used fMRI to measure brain activity associated with three stages of the placebo response: before, during and after the sham treatment, while participants anticipated and experienced brief laser pain. In the control session participants were explicitly told that the treatment was inactive. The sham treatment group reported a significant reduction in pain rating (p = 0.012). Anticipatory brain activity was modulated during placebo conditioning in a fronto-cingulate network involving the left dorsolateral prefrontal cortex (DLPFC), medial frontal cortex and the anterior mid-cingulate cortex (aMCC). Identical areas were modulated during anticipation in the placebo analgesia phase with the addition of the orbitofrontal cortex (OFC). However, during altered pain experience only aMCC, post-central gyrus and posterior cingulate demonstrated altered activity. The common frontal cortical areas modulated during anticipation in both the placebo conditioning and placebo analgesia phases have previously been implicated in placebo analgesia. Our results suggest that the main effect of placebo arises from the reduction of anticipation of pain during placebo conditioning that is subsequently maintained during placebo analgesia.
Keywords: Placebo analgesia, Placebo, fMRI, Laser, Conditioning
This work was funded by the ARC (UK) Arthritis Research Campaign.
1. Introduction
Belief in the efficacy of a treatment and the context in which it is administered play a key role in the subsequent expectation and perception of pain relief [2,7,16]. During experimental placebo analgesia, placebo conditioning is commonly used to enhance the expectation of the effectiveness of a sham treatment [28,39,40,44].
Previous imaging studies of pain modulation by placebo have shown brain activations in the rostral anterior cingulate, insula, thalamus and mid-brain regions [4,26,43,49]. During the anticipation reduced pain activations were seen in the orbitofrontal cortex (OFC) and dorsolateral prefrontal cortex (DLPFC) [43]. It was suggested that one of the main effects of placebo analgesia may be to alter the anticipation of pain [43]. However, so far the brain processes that occur during placebo conditioning have not been investigated.
The term conditioning is commonly used in studies of placebo analgesia [39,41,44,45] to describe the association between covert reduction in the pain stimulus with the application of a sham analgesic treatment so that the participant believes an active treatment is involved. However, it can be argued that this is not classical conditioning [30] as we know it but more a test of the effects of analgesic suggestion.
In order to take this further we performed an experiment to determine if there are common brain areas that are modulated throughout both the placebo conditioning and the placebo analgesia period. In the current study we used fMRI in conjunction with an established method of experimental placebo analgesia [28,41,44,45] to image three stages of placebo induction: pre-treatment (or pre-conditioning); sham treatment paired with pain reduction (placebo conditioning); and post-conditioning. We intentionally focussed on the pain anticipation period prior to delivery of a laser stimulus and compared changes in the fMRI signal during placebo conditioning with those in the post-conditioning period. We included a separate control session for comparison, where the same participants had no expectation of analgesia. The sessions were identical in terms of their sensory content, differing only in the information given to the participants about the expected treatment in the placebo session. This session also allowed us to control for habituation effects.
2. Methods
2.1. Participants
Eleven healthy, (six female, five male) neurologically normal, right handed participants (age range 19–36 years) gave their informed consent to take part in the study. Participants had two experimental sessions; sham treatment (placebo) session followed by a control session. They were informed that the aim of the study was to measure brain activity associated with brief moderately painful laser heat pulses and how this sensation is affected by the application of either a local anaesthetic cream or an inactive cream. Participants were financially rewarded for taking part in the study and participants who had previously used local anaesthetic creams, or who had taken part in studies involving the investigation of placebo responses were excluded from the study. The study was approved by Oxford NHS Local Research Ethics Committee.
2.2. Apparatus and materials
2.2.1. Laser stimuli
The pain stimuli were delivered by a neodymium yttrium aluminium perovskite (Nd:YAP) laser wavelength 1.34 μm (Electronical Engineering Florence Italy) (pulse duration 4 ms, beam diameter 6 mm) to a 5 × 3 cm stimulation area marked on the dorsal surface of the right forearm. Stimuli were randomly moved around the stimulation area, in order to minimise sensitisation and/or habituation. For each stimulation block, 15 laser stimuli were delivered to the forearm.
Participants were trained to rate the pain of each laser stimulus using a 0–10 pain scale, where 0 indicates no sensation, 4 indicates just painful and 10 indicates worse imaginable pain possible. This scale allowed the participants to rate stimuli they perceived as non-painful. At the start of the study, we determined the laser energies corresponding to each subject’s non-painful level 3 (2.5 ± 1.2 J) and moderately painful level 7 (4 ± 1 J) using a series of stimuli of ascending intensities. We used the mean laser energy for the moderately painful level 7 and non-painful level 3 to check for reproducibility.
2.3. Design
2.3.1. Sham treatment session
The participants’ right arm was conditioned in the sham treatment session but not in the control session. In the sham treatment session participants were told that they may receive either a local anaesthetic or an inactive cream. In fact, all participants received an inactive cream. The experiment was divided into three blocks. The cream was applied between blocks 1 and 2. In this context conditioning is defined as turning the pain stimulus down to a non-painful level, so that participants believed that a local anaesthetic cream had been applied to the skin.
2.3.1.1. Block 1 (pre-conditioning)
Prior to the application of the cream, participants received fifteen moderately painful (level 7) laser stimuli to each arm. They rated the level of pain of each stimulus.
2.3.1.2. Cream application
Inactive aqueous cream was applied to the entire laser stimulation area on the right forearm. The area was then covered with an occlusive dressing and left in place for 10 min. Participants were told that the cream would take effect during this time. After this the dressing was removed and the cream wiped off.
2.3.1.3. Block 2 (conditioning)
During this block the intensity of the laser stimuli was reduced to each participant’s non-painful level (level 3); participants were not told that the intensity had been decreased. They received 15 laser stimuli to the forearm, and rated the level of pain of each stimulus.
2.3.1.4. Block 3 (post-conditioning)
This block was identical to the pre-conditioning block; 15 moderately painful (level 7) laser stimuli were delivered to the forearm and participants rated the level of pain of each stimulus.
2.3.2. Control session
Approximately 1 week after the sham treatment session participants underwent the same procedure as in the sham treatment session, but with different information. Participants were told that an inactive cream would be applied to the right forearm and that, in block 2, the pain stimulus would be reduced to their predetermined non-painful level. Participants rated the level of pain of each stimulus. The reason that the control session was always after the sham treatment session was to maximise subject’s belief in the analgesic effects of the cream. The energy of the laser stimuli during block 1 and block 3 for the sham treatment session and control session was identical (the participants’ predetermined level 7 of pain).
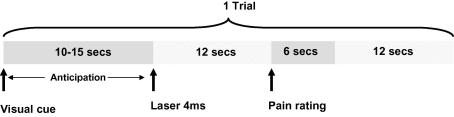
Participants remained inside the scanner during both the sham treatment and control sessions and during the three experimental blocks. Three fMRI scans were performed one for each of the experimental blocks. The stimulus procedure can be seen in Fig. 1.
Fig. 1.
Schematic of study design: each of the two scanning sessions consisted of three scans (preconditioning, conditioning, and post-conditioning). Each scan consisted of 15 trials. The laser stimulus was delivered to the right arm, and to rate the pain participants moved a cursor along a projected 0–10 scale.
2.4. Functional data acquisition and analysis
The data were collected using a 3-T (Oxford Magnet Technologies) MRI research scanning system (Varian/Siemens). Functional images were acquired using a gradient-echo, Echo Planar Imaging (EPI) sequence (TR = 3000 ms, TE = 30 ms). The whole brain was imaged by 21 contiguous axial slices 7 mm thick with an in-plane voxel size of 4 × 4 mm (64 × 64 image matrix). A T1 weighted high resolution structural anatomical scan was also taken of the whole brain with 64 axial slices, 3 mm thick and an in-plane voxel size of 1 × 1 mm (256 × 256 image matrix).
Initial analysis of fMRI images to identify regions exhibiting significant changes in BOLD signal was carried out in a multistage process using FEAT (FMRI Expert Analysis Tool) Version 5.63, part of FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl; [33]). The following pre-statistics processing was applied; motion correction using MCFLIRT [15]; non-brain removal using BET [32]; spatial smoothing using a Gaussian kernel of FWHM 5 mm; mean-based intensity normalisation of all volumes by the same factor; high pass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma = 50.0 s). Time-series statistical analysis was carried out using FILM with local autocorrelation correction [47]. Z (Gaussianised T/F) statistic images were thresholded using clusters determined by Z > 2.3 and a (corrected) cluster significance threshold of P = 0.05 [48]. Registration to high resolution and/or standard images was carried out using FLIRT [17].
Higher-level analysis was carried out using FLAME (FMRIB’s Local Analysis of Mixed Effects) [1,46]. Z (Gaussianised T/F) Statistical images were thresholded using clusters determined by Z > 1.9 and a (corrected) cluster significance threshold of P = 0.05 [48].
The use of a combined height and cluster threshold is a standard and generally accepted method to solve the problem of multiple comparisons with functional imaging data [11,12,48]. The combined height and cluster threshold is the default option with FSL, and is consequently commonly adopted in papers using FSL for data analysis, but similar combinations have also been adopted by investigators using other analysis packages such as SPM [13,14], AFNI [25] and Brain Voyager [24].
4. Results
4.1. Behavioural results
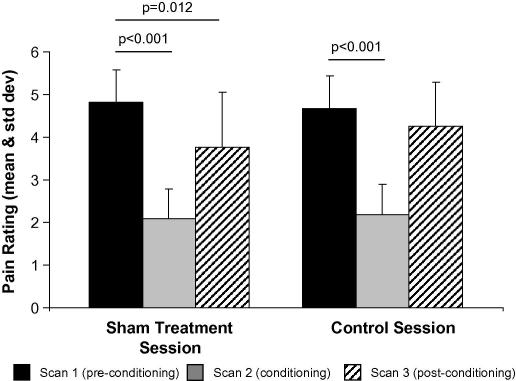
An ANOVA of the pain ratings within the pre-conditioning and post-conditioning blocks between the sham treatment and control sessions revealed a significant effect of experimental block (F1,10 = 13.8 p = 0.005) and a session × block interaction (F1,10 = 12.9 p = 0.006). This demonstrates that the change in pain rating from the pre-stimulus reduction to the post-stimulus reduction block is significantly different between the two sessions.
Paired t-tests for the control session showed no significant changes in pain ratings between the pre- and post-conditioning blocks (Fig. 2). During the conditioning block when the stimulus intensity was turned down to the participants previously determined non-painful level 3, there was a significant reduction in pain rating from 4.68 ± 0.76 (using the 0–10 pain scale) in the pre-conditioning block to 2.19 ± 0.70 in the conditioning block (t(10) = 8.67 p < 0.001).
Fig. 2.
Mean and standard deviation of pain ratings (0–10 pain scale) during the placebo and control sessions. The same laser energy was applied in the pre-conditioning and post-conditioning blocks in both sessions, set at each individual’s moderately painful level. Mean rating during the placebo session: pre-conditioning 4.82 (0.77), conditioning 2.09 (0.69), post-conditioning 3.77 (1.29), and during the control session: preconditioning 4.68 (0.76), conditioning 2.19 (0.70), post-conditioning 4.26 (1.03).
In the sham treatment session, pain ratings were significantly reduced between the pre-conditioning, conditioning and post-conditioning blocks. For example, in the pre-conditioning block for the sham treatment session the mean pain (±1 standard deviation) rating was 4.82 ± 0.77 (using a 0–10 pain scale). When the stimulus intensity was turned down during the conditioning block the participants perceived that stimulus pain rating reduced significantly to 2.09 ± 0.69 (t(10) = 12.5 p < 0.001).
During the post-conditioning block, when the stimulus intensity was turned back up to the same level used in the pre-conditioning block, the participants mean pain (±1 standard deviation) rating was 3.77 ± 1.29 compared to 4.82 ± 0.77. This constituted a significant placebo-induced reduction (mean 1.05, on the 0–10 pain scale) in pain rating compared to the pre-conditioning block (t(10) = 3.05 p < 0.012).
4.2. fMRI results
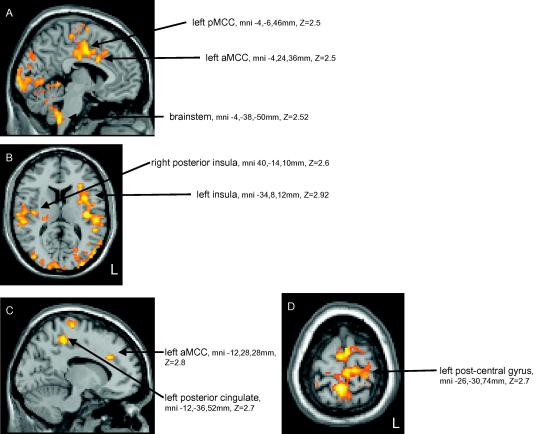
To show the brain regions that correlate with the intensity of pain stimulation, we calculated a contrast between the painful (pre-conditioning scan) and non-painful (control conditioning scan) laser stimuli during the control session (Z > 1.8 p = 0.05 corrected). Significant activations were seen in areas of the “pain matrix” including; bilateral cerebellum, bilateral insula, left post-central gyrus, brainstem and ACC (Fig. 3A and B).
Fig. 3.
(A and B) Sites of increased activation in brain regions that correlate with the intensity of pain stimulation, contrast between the painful (pre-conditioning scan) and non-painful (control conditioning scan) laser stimuli during the control session (Z > 1.8 p = 0.05 corrected for multiple comparisons). Significant activations were seen in areas of the “pain matrix” including; bilateral cerebellum, bilateral insula, left post-central gyrus, brainstem and ACC. (C and D) Sites of increased brain activation during placebo analgesia co-varied with measures of reported pain relief. The magnitude of reduction between control and sham treatment sessions co-varied with the magnitude of reduction in neural activity (control minus sham treatment scans for the post-conditioning blocks during painful stimulation). These structures included the following areas within the “pain matrix”; the left aMCC and PCC and left post-central gyrus (Z > 1.9 p = 0.05 corrected for multiple comparisons).
To exclude non-specific between-session changes in the responsiveness of the pain matrix, we did a comparison of the pattern of brain activity associated with the painful stimulus:
Sham-treatment (pre-conditioning block minus conditioning block) minus control (pre-conditioning block minus conditioning block).
No significant differences were seen.
4.3. Activation sites during placebo analgesia
We assessed the placebo effect based on the behavioural results calculated as the difference between the average rating of pain in the placebo and control sessions (as in Wager et al. [43]). This allowed us to examine the correlation between measures of reported pain relief and the corresponding neural responses. We found that the magnitude of reduction between control and sham treatment sessions correlated with the magnitude of reduction in neural activity (control minus sham treatment scans for the post-conditioning blocks during painful stimulation). These structures included the following areas within the “pain matrix”; the left aMCC and PCC and left post-central gyrus (Z > 1.9 p = 0.05 corrected) (Fig. 3C and D).
4.4. The anticipation of reduced pain
The effects of placebo analgesia on the anticipation phase of the laser stimulus were then analysed. We compared the following contrast for the sham treatment session with the same during the control session.
Sham treatment: (post-conditioning block minus pre-conditioning block) minus control: (post-conditioning block minus pre-conditioning block).
There were significant increases in neuronal activity during the anticipation phase in the sham treatment session compared to those in the control session, in the left DLPFC (BA 9), bilateral OFC (BA11), and the left medial frontal cortex (BA 8) (Table 1b and Fig. 4).
Table 1.
(a) Sites of increased activation for the subtraction contrast conditioning scan minus the preconditioning scan during the anticipation phase of the sham treatment session compared to the same in the control session. (b) Sites of increased activation for the subtraction contrast post-conditioning scan minus the preconditioning scan during the anticipation phase of the placebo session compared to the same in the control session. MNI (mm) co-ordinate, laterality and peak Z score of the maximum activating voxel in each cluster are shown. Activations are determined by clusters greater than Z > 1.9, p = 0.05 (corrected for multiple comparisons).
| Region (BA) | MNI coordinate x, y, z (nm) |
Z score | |
|---|---|---|---|
| Right | Left | ||
| (a) Anticipation [sham treatment (conditioning–preconditioning) minus control (conditioning–preconditioning)] | |||
| aMCC | 4, −22, 42 | −6, 32, 24 | 2.91 |
| DLPFC (8) | −26, 52, 18 | 2.91 | |
| MFC (10) | −6, 44, 34 | 2.91 | |
| dPCC (24) (23) | −2, −32, 32 | 2.9, 2.83 | |
| Occipital cortex (19) | −20, −62, 2 | 2.67 | |
| Retrosplenial cortex (30) | −8, −48, 16 | 2.52 | |
| Precuneus (7) | −8, −58, 36 | 2.52 | |
| (b) Anticipation [sham treatment (post-conditioning–preconditioning) minus control (post-conditioning–preconditioning)] | |||
| DLPFC (9) | 12, 38, −12 | −24, 52, 18 | 2.9 |
| MFC (8) | −6, 44, 34 | 2.91 | |
| pACC border (32) | −8, 42, −10 | 2.92 | |
| aMCC | −6, 32, 24 | 2.89 | |
| OFC (11) | −10, 40, −12 | 2.8 | |
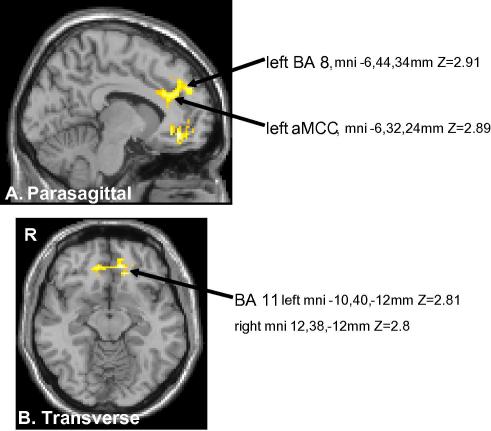
Fig. 4.
Significant activations for the subtraction contrast post-conditioning scan minus the preconditioning scan during the anticipation phase of the sham treatment session compared to the same in the control session [e.g., sham treatment (post-conditioning–preconditioning) minus control (post-conditioning–preconditioning)]. The map was cluster-based thresholded at Z > 1.9, p = 0.05 (corrected for multiple comparisons) and the images are shown in axial and sagittal orientations and radiological convention (right side of the brain on the left side of the picture).
There were two cingulate activation sites in the left hemisphere. One was in a ventral part of pACC and included areas a24′ and 32′ and extended into area 9. The second site was in the rostral part of anterior MCC (aMCC; Vogt et al. [37,38]) and included areas 24′ and 32′.
4.5. The effect of conditioning on the anticipation of pain
In order to measure the effect of conditioning, i.e., the coupling of the application of the sham treatment with a reduction in the painful laser stimulus, we compared the sham treatment session with the control session using the following contrast:
Sham treatment session (conditioning block minus pre-conditioning block) minus control session (conditioning block minus pre-conditioning block).
Areas activated (Table 1a) include left anterior MCC, left DLPFC, bilateral dPCC, left occipital cortex, left retrosplenial cortex, and left precuneus.
4.6. Overlapping areas of activation during conditioning and post-conditioning
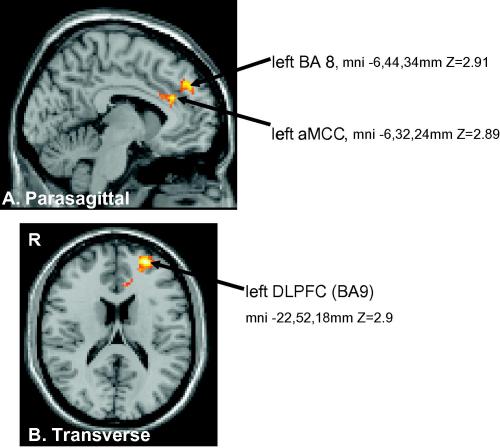
We used an inclusive mask to demonstrate overlapping areas of activation during anticipation in the conditioning and post-conditioning contrasts discussed previously. The overlapping areas are shown in Fig. 5. These included the left DLPFC (mni, x, y, z = −22, 52, 18 mm, BA 9), left BA10 (x, y, z = −6, 44, 34 mm) and left aMCC (mni −6, 32, 24 mm). Z > 1.9 p = 0.05 corrected. The latter site included areas 24′ and 32′.
Fig. 5.
Common brain areas activated during pain anticipation in both the placebo conditioning (Table 1a) and post-conditioning (Table 1b) stages compared to the pre-treatment stage. DLPFC (left dorsolateral prefrontal cortex) (mni −22, 52, 18, BA 9), left BA 8, mni −6, 44, 34 and left aMCC mni −6, 32, 24. The images are shown in axial and sagittal orientations and radiological convention (right side of the brain on the left side of the picture).
5. Discussion
In this study we investigated the changes in brain activity during placebo conditioning and the two components of placebo analgesia, anticipation and nociception. This study does not address the mechanisms involved in classical Pavlovian conditioning but rather tests for the effects of analgesic suggestion.
Anticipation of pain reduction during placebo conditioning (i.e., sham treatment) and post-conditioning activated fronto-cingulate structures including DLPFC, aMCC and MFC. Altered activity was observed during the nociceptive component of placebo analgesia in a network of structures that included the aMCC, SI and PCC, suggesting a hierarchy of cortical and subcortical networks may operate to maintain adaptive responses after placebo conditioning. aMCC appears to be common to both networks.
We induced placebo analgesia by associating a sham treatment with pain reduction and used fMRI to image responses before, during and after the sham treatment. We compared brain activation during pain anticipation at each stage. To control for habituation, we used a separate control session where the same participants had no expectation of treatment or analgesia as suggested by Wager et al. [42]. This contrasts with a design where a control (untreated) site is used in the same imaging session. Although there is evidence that site-specific conditioning cues lead to placebo effects in those sites [3], the absence of site-specific cues can result in placebo effects in unconditioned sites [44,45]. This design allows us to optimally control for habituation. The results should therefore provide a more accurate representation of the top-down effects of conditioning and subsequent placebo analgesia than experiments where habituation is not controlled for.
The treatment and control sessions were identical in terms of their sensory content, differing only in the information on the expected treatment in the placebo conditioning block of the treatment session. Therefore, the effect specifically observed in the treatment session would likely involve primarily suggestion-related effects consolidated by the conditioning procedure while the control session may involve conditioning effects in spite of no analgesic suggestion, i.e., learnt behaviour that creams are medicinal and have a soothing effect on the skin.
The design also allowed us to contrast the anticipation period during the placebo conditioning (when we administered the sham treatment and reduced the laser energy) with that of the (painful) pre-conditioning stage with the control session. In the corresponding stage of the control session subjects were informed that the treatment was inactive and laser energy reduced. In this contrast, the BOLD response during conditioning showed increased activity in the left aMCC, the left medial frontal cortex, left DLPFC, but not in the OFC (Table 1a).
Anterior MCC [37] was commonly activated during reduced anticipation in both conditioning and post-placebo conditioning (Fig. 5) and deactivated during reduced nociceptive processing of placebo analgesia (Fig. 3C). This may be the area of the brain that maintains altered brain responses from the reduction in anticipation to the reduced pain experience during the placebo process. It is intriguing that this area has also been found to be commonly activated during placebo and opiate analgesia and has been identified as a key site for placebo-mediated release of endogenous opioid peptides [6,26,43,49].
During the placebo conditioning block (when the pain stimulus was covertly reduced) learning occurs when the subject associates the reduction in pain perception with the sham treatment. During the anticipation phase of placebo conditioning we saw significant activations in the aMCC, PFC (DLPFC, MFC) and dPCC. The PCC is involved in visuospatial orientation and assessment of self-relevant sensation and the more dorsal PCC is associated with orientation of the body to both innocuous and noxious somatosensory stimuli ([37]. These combined are essential components of the learning process.
During the post-conditioning block when the laser pain stimulus was turned back up to the subjective pain level used in the pre-conditioning block the subjects perceived less pain than the pre-conditioning block. During the anticipation phase we saw significant activations in the same areas as during conditioning (aMCC, PFC (DLPFC, MFC) and dPCC) with the addition of the OFC. Modulation of activity in the MCC has been associated with the affective aspects of pain [20,27,29,37] and pain intensity coding [5,17,18,43]. Previous results demonstrated increases in aMCC activity during the anticipation component of placebo analgesia suggesting inhibition of the affective components of nociceptive processing [17,22,43]. The anticipation of reduced pain and actual perception of reduced pain could serve as the foundation of a self-reinforcing feedback loop underpinned by the previously associated learning.
Furthermore, for a placebo treatment to be effective, the memory of the effectiveness of the sham treatment developed during the (learning) conditioning phase of the experiment must be retrieved and matched with the incoming sensory information. The PFC with its role in memory retrieval and working memory [31], may be maintaining the belief in the treatment. Craggs et al. [10] suggests that during placebo analgesia there is an increased influence of the DLPFC on the ACC which involves the recall and, perhaps more importantly maintenance of the effectiveness of the placebo treatment. If the matching continues to confirm the expectation of an analgesia effect, this could serve as the foundation of a self-reinforcing feedback loop underpinning the placebo effect [9,34–36]. Others have also reported the involvement of the DLPFC for context maintenance and working memory [8] and expectation of pain relief [23].
The aMCC, MFC and DLPFC were commonly activated during the anticipation phase of both placebo conditioning and post-conditioning. It is possible that during the anticipation phase during placebo conditioning the reduced intensity coding of pain in the aMCC drives associated learning via activation of PFC with an outcome of reward in the form of reduced pain due to the sham treatment. During the anticipation phase of the post-conditioning block, activation of PFC may represent retrieval from memory of the effectiveness of the sham treatment. This may in turn reinforce altered reappraisal strategies leading to reduced pain perception and intensity coding in the aMCC.
During the nociceptive component of placebo analgesia (Fig. 3C and D, control >placebo) we showed a reduction in activation of the “pain matrix” (left aMCC, left SI and left PCC) similar to other placebo analgesia studies [17,19,21,26,43]. SI and the ACC are associated with the encoding of differing pain intensity levels [27]. Modulation of activity in the MCC has been associated with the affective aspects of pain, [20,27,29,37] and pain intensity coding [5,17,18,43]. Decreases in brain activity in the MCC during placebo analgesia may indicate inhibition of the affective components of pain due to reduced intensity coding [17,43].
Our results show that aMCC is involved in both the anticipation of reduced pain during placebo conditioning and placebo analgesia and also in reduced perception of pain during placebo analgesia. The deactivation of the aMCC during reduced nociceptive processing of placebo analgesia is consistent with its involvement in pain intensity coding and affect. The perception of reduced pain is likely to maintain the self-reinforcing feedback loops within fronto-cingulate circuits.
In conclusion, this study provides evidence for a network of pre-frontal and mid-cingulate cortices that is involved in the maintenance of altered anticipation of pain during placebo conditioning and post-conditioning. We argue that it is the modulation of this network that results in the adaptive responses observed within the pain matrix and associated structures during placebo analgesia. The aMCC appears to be the crucial cortical link between the modulation of anticipation and maintenance of placebo analgesia.
Acknowledgements
We thank the reviewers for their constructive comments, some of their text has been included in the manuscript. There are no conflicting interests associated with this manuscript.
References
- 1.Beckmann C.F., Jenkinson M., Smith S.M. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- 2.Benedetti F. How the doctor’s words affect the patient’s brain. Eval Health Prof. 2002;25:369–386. doi: 10.1177/0163278702238051. [DOI] [PubMed] [Google Scholar]
- 3.Benedetti F., Arduino C., Amanzio M. Somatotopic activation of opoid systems by target directed expectations of analgesia. J Neurosci. 1999;19:3639–3648. doi: 10.1523/JNEUROSCI.19-09-03639.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bingel U., Lorenz J., Schoell E., Weiller C., Buchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 5.Buchel C., Bornhovd K., Quante M., Glauche V., Bromm B., Weiller C. Dissociable neural responses related to pain intensity, stimulus intensity, and stimulus awareness within the anterior cingulate cortex: a parametric single-trial laser functional magnetic resonance imaging study. J Neurosci. 2002;22:970–976. doi: 10.1523/JNEUROSCI.22-03-00970.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casey K.L., Svensson P., Morrow T.J., Raz J., Jone C., Minoshima S. Selective opiate modulation of nociceptive processing in the human brain. J Neurophysiol. 2000;84:525–533. doi: 10.1152/jn.2000.84.1.525. [DOI] [PubMed] [Google Scholar]
- 7.Charron J., Rainville P., Marchand S. Direct comparison of placebo effects on clinical and experimental pain. Clin J Pain. 2006;22:204–211. doi: 10.1097/01.ajp.0000161526.25374.e5. [DOI] [PubMed] [Google Scholar]
- 8.Cohen J.D., Perlstein W.M., Braver T.S., Nystrom L.E., Noll D.C., Jonides J., Smith E.E. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- 9.Craggs J., Price D.D., Perlestein W., Verne G.N., Robinson M.E. The dynamic mechanisms of placebo induced analgesia: evidence of sustained and transient regional involvement. Pain. 2008;139:660–669. doi: 10.1016/j.pain.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craggs J.G., Price D.D., Verne G.N., Perlstein W.M., Robinson M.M. Functional brain interactions that serve cognitive-affective processing during pain and placebo analgesia. Neuroimage. 2007;38:720–729. doi: 10.1016/j.neuroimage.2007.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forman S.D., Cohen J.D., Fitzgerald M., Eddy W.F., Mintun M.A., Noll D.C. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 12.Friston K., Worsley K., Frackowiak R., Mazziotta J., Evans A. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1992;1:214–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- 13.Gu X., Han S. Attention and reality constraints on the neural processes of empathy for pain. Neuroimage. 2007;36:256–267. doi: 10.1016/j.neuroimage.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 14.Gu X., Han S. Neural substrates underlying evaluation of pain in actions depicted in words. Behav Brain Res. 2007;181:218–223. doi: 10.1016/j.bbr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 16.Kaptchuk T.J., Goldman P., Stone D.A., Stason W.B. Do medical devices have enhanced placebo effects? J Clin Epidemiol. 2000;53:786–792. doi: 10.1016/s0895-4356(00)00206-7. [DOI] [PubMed] [Google Scholar]
- 17.Kong J., Kaptchuk T.J., Polich G., Kirsch I., Gollub R.L. Placebo analgesia: findings from brain imaging studies and emerging hypotheses. Rev Neurosci. 2007;18:173–190. doi: 10.1515/revneuro.2007.18.3-4.173. [DOI] [PubMed] [Google Scholar]
- 18.Kong J., White N.S., Kwong K.K., Vangel M.G., Rosman I.S., Gracely R.H., Gollub R.L. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum Brain Mapp. 2006;27:715–721. doi: 10.1002/hbm.20213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong J., Gollub R.L., Rosman I.S., Webb J.M., Vangel M.G., Kirsch I., Kaptchuk T.J. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci. 2006;26:381–388. doi: 10.1523/JNEUROSCI.3556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kulkarni B., Bentley D.E., Elliott R., Youell P., Watson A., Derbyshire S.W., Frackowiak R.S., Friston K.J., Jones A.K. Attention to pain localization and unpleasantness discriminates the functions of the medial and lateral pain systems. Eur J Neurosci. 2005;21:3133–3142. doi: 10.1111/j.1460-9568.2005.04098.x. [DOI] [PubMed] [Google Scholar]
- 21.Lieberman M.D., Jarcho J.M., Berman S., Naliboff B.D., Suyenobu B.Y., Mandelkern M., Mayer E.A. The neural correlates of placebo effects: a disruption account. Neuroimage. 2004;22:447–455. doi: 10.1016/j.neuroimage.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 22.Lieberman M.D., Jarcho J.M., Berman S., Naliboff B.D., Suyenobu B.Y., Mandelkern M., Mayer E.A. The neural correlates of placebo effects: a disruption account 1. NeuroImage. 2004;22:447–455. doi: 10.1016/j.neuroimage.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 23.Lorenz J., Minoshima S., Casey K.L. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126:1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- 24.Morrison I., Peelen M.V., Downing P.E. The sight of others’ pain modulates motor processing in human cingulate cortex. Cereb Cortex. 2007;17:2214–2222. doi: 10.1093/cercor/bhl129. [DOI] [PubMed] [Google Scholar]
- 25.Peng D.L., Ding G.S., Perry C., Xu D., Jin Z., Luo Q., Zhang L., Deng Y. fMRI evidence for the automatic phonological activation of briefly presented words. Brain Res Cogn Brain Res. 2004;20:156–164. doi: 10.1016/j.cogbrainres.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Petrovic P., Ingvar M. Placebo and opioid analgesia imaging a shared neuronal network. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 27.Peyron R., Laurent B., Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Clin Neurophysiol. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 28.Price D.D., Milling L.S., Kirsch I., Duff A., Montgomery G.H., Nicholls S.S. An analysis of factors that contribute to the magnitude of placebo analgesia in an experimental paradigm. Pain. 1999;83:147–156. doi: 10.1016/s0304-3959(99)00081-0. [DOI] [PubMed] [Google Scholar]
- 29.Rainville P. Brain mechanisms of pain affect and pain modulation. Curr Opin Neurobiol. 2002;12:195–204. doi: 10.1016/s0959-4388(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 30.Rescorla R.A. Pavlovian conditioning, its not what you think it is. Am Psychol. 1988;43:151–160. doi: 10.1037//0003-066x.43.3.151. [DOI] [PubMed] [Google Scholar]
- 31.Rolls E.T. Memory systems in the brain. Annu Rev Psychol. 2000;51:599–630. doi: 10.1146/annurev.psych.51.1.599. [DOI] [PubMed] [Google Scholar]
- 32.Smith S.M. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H., Bannister P.R., De L.M., Drobnjak I., Flitney D.E., Niazy R.K., Saunders J., Vickers J., Zhang Y., De S.N., Brady J.M., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 34.Vase L., Robinson M.E., Verne G.N., Price D.D. The contributions of suggestion, desire, and expectation to placebo effects in irritable bowel syndrome patients: an empirical investigation. Pain. 2003;105:17–25. doi: 10.1016/s0304-3959(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 35.Vase L., Robinson M.E., Verne G.N., Price D.D. Increased placebo analgesia over time in irritable bowel syndrome (IBS) patients is associated with desire and expectation but not endogenous opioid mechanisms. Pain. 2005;115:338–347. doi: 10.1016/j.pain.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 36.Verne G.N., Himes N.C., Robinson M.E., Gopinath K.S., Briggs R.W., Crosson B., Price D.D. Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain. 2003;103:99–110. doi: 10.1016/s0304-3959(02)00416-5. [DOI] [PubMed] [Google Scholar]
- 37.Vogt B.A. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vogt B.A., Berger G.R., Derbyshire S.W. Structural and functional dichotomy of human midcingulate cortex. Eur J Neurosci. 2003;18:3134–3144. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voudouris N.J., Peck C.L., Coleman G. Conditioned placebo responses. J Pers Soc Psychol. 1985;48:47–53. doi: 10.1037//0022-3514.48.1.47. [DOI] [PubMed] [Google Scholar]
- 40.Voudouris N.J. Conditioned response models of placebo phenomena: further support. Pain. 1989;38:109–116. doi: 10.1016/0304-3959(89)90080-8. [DOI] [PubMed] [Google Scholar]
- 41.Voudouris N.J. The role of conditioning and verbal expectancy in the placebo response. Pain. 1990;43:121–128. doi: 10.1016/0304-3959(90)90057-K. [DOI] [PubMed] [Google Scholar]
- 42.Wager T.D., Matre D., Casey K.L. Placebo effects in laser-evoked pain potentials. Brain Behav Immun. 2006;20:219–230. doi: 10.1016/j.bbi.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wager T.D., Rilling J.K., Smith E.E., Sokolik A., Casey K.L., Davidson R.J., Kosslyn S.M., Rose R.M., Cohen J.D. Placebo-induced changes in fMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 44.Watson A., El-Deredy W., Bentley D.E., Vogt B.A., Jones A.K.P. Categories of placebo response in the absence of site-specific expectation of analgesia. Pain. 2006;126:115–122. doi: 10.1016/j.pain.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 45.Watson A., El-Deredy W., Vogt B.A., Jones A.K. Placebo analgesia is not due to compliance or habituation: EEG and behavioural evidence. Neuroreport. 2007;18:771–775. doi: 10.1097/WNR.0b013e3280c1e2a8. [DOI] [PubMed] [Google Scholar]
- 46.Woolrich M.W., Behrens T.E., Beckmann C.F., Jenkinson M., Smith S.M. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 47.Woolrich M.W., Ripley B.D., Brady M., Smith S.M. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- 48.Worsley K.J., Evans A.C., Marrett S., Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- 49.Zubieta J.K., Bueller J.A., Jackson L.R., Scott D.J., Xu Y., Koeppe R.A., Nichols T.E., Stohler C.S. Placebo effects mediated by endogenous opioid activity on {micro}-opioid receptors. J Neurosci. 2005;25:7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]