Figure 1.
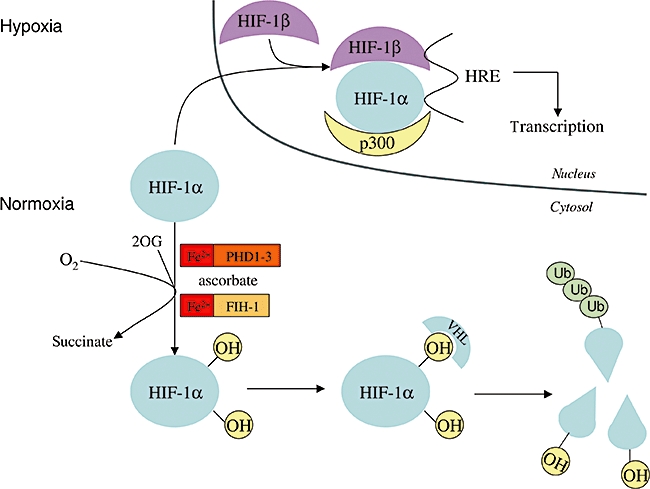
Role of ascorbate in the destruction of HIF-1α in normoxia. During hypoxia, HIF-1α enters the nucleus, forms a dimer with HIF-1β and the dimer binds to DNA through the hypoxia response element (HRE). In this way, transcription of oxygen-dependent genes is initiated. In normoxia, in the presence of oxygen and ascorbate, two proline residues and one asparagine residue of HIF-1α are hydroxylated by Fe(II) and 2-oxo-glutarate (2OG)-dependent dioxygenases, as prolyl hydroxylase domain family members, and by asparaginyl hydroxylase factor inhibiting HIF-1, respectively. Hydroxylation of prolyl residues results in the binding of the von Hippel–Lindau protein, which is part of the E3 ubiquitine ligase complex. This is the precondition for ubiquitination, which leads to the degradation of HIF-1α. Alternatively, Asn-hydroxylation prevents the binding of p300 transcription coactivator protein to HIF-1α. Thus, it inhibits transactivation of HIF-1α and the transcription of oxygen-dependent genes.
