Figure 3. Mutations of the acid-dipole interaction disrupt the Spt4-Spt5 complex.
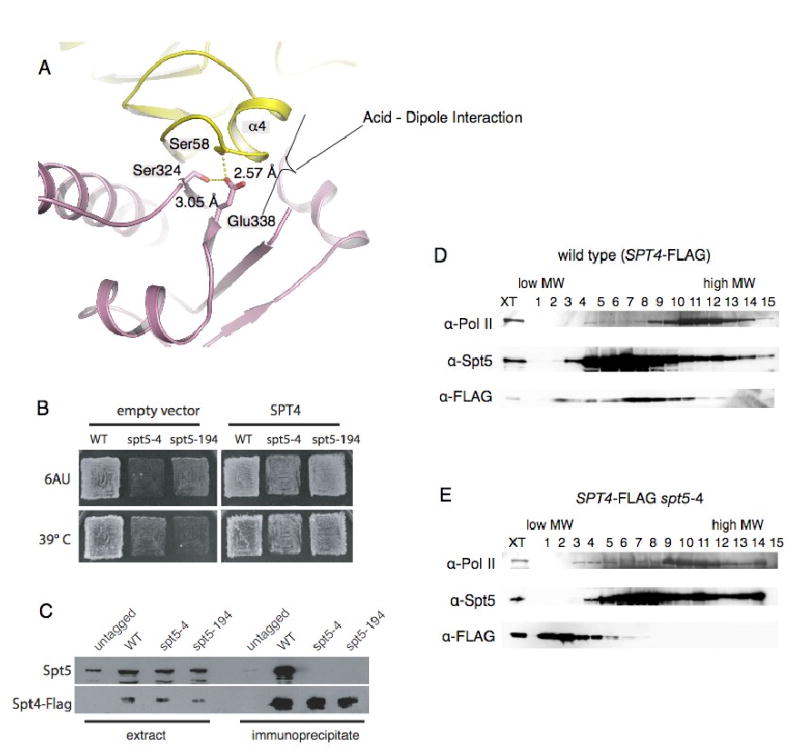
(A) Conserved acid--α-helix dipole interaction between Helix_α4 of Spt4 and Glu338 of Spt5NGN. The carboxyl of Glu338 is also fixed by two hydrogen-bonds with Spt4_Ser58 and Spt5_Ser324. The coloring is the same as in Figure 2. (B) Suppression of spt5-4 and spt5-194 by overexpression of Spt4. The indicated strains were transformed with either empty vector or a high copy Spt4 plasmid and replica plated to YPD media at 39° C or SC-ura media with 50 microgram per ml 6-azauracil at 30° C. (C) Co-immunoprecipitation of Spt4-Spt5 from extracts of Spt5, Spt5-4 or Spt5-194 cells. (D, E) The spt5-4 mutation disrupts the Spt4-Spt5 complex; Extracts of cells expressing wild type Spt5 or Spt5-4 and flag-tagged Spt4 were fractionated on 10-30% sucrose gradients. Fractions were separated on SDS gels and blotted with the indicated anti-sera.
