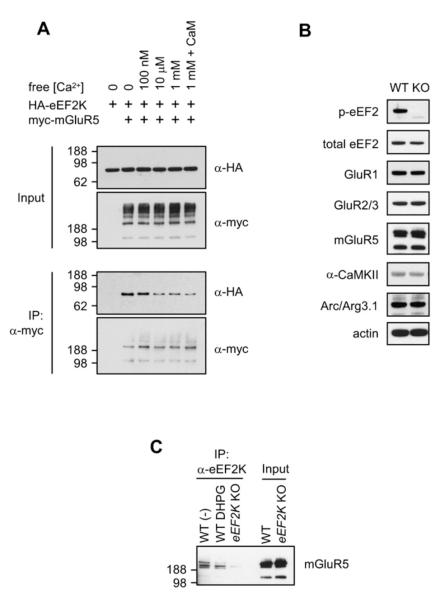
Figure 4. Dynamic Interaction of eEF2K and mGluR5.
(A) Calcium dissociates eEF2K from mGluR5. HEK293T cells were transfected with HA-eEF2K with or without myc-mGluR5, and cells were harvested with lysis buffer without calcium or containing various concentrations of free calcium. Calmodulin (CaM) (25 μg/ml) was also added to the lysis buffer as indicated. Binding was decreased at [Ca2+] higher than 10 μM.
(B) Phospho-eEF2 was not detected in the hippocampus of eEF2K KO, while the level of total eEF2, GluR1, Glur2/3, mGluR5, α-CaMKII, Arc/Arg3.1, and actin was not altered in eEF2K KO mice compared to WT littermate controls.
(C) Synaptoneurosomes, prepared from the forebrain of eEF2K KO and WT mice, were stimulated with vehicle or DHPG for 20 min. Synaptoneurosomes were then lysed and immunoprecipitated with anti-eEF2K antibody. mGluR5 co-IPed with eEF2K only in WT samples. Stimulation of synaptoneurosomes with DHPG decreased the co-IP of mGluR5.