Table 1.
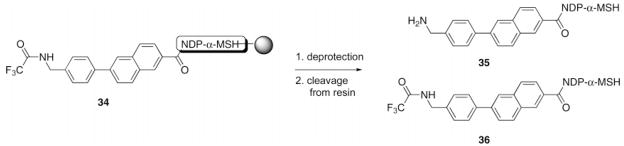
Optimization of N-TFA Cleavage Using Resin 34a
 | |||||
|---|---|---|---|---|---|
| yields (%)b |
|||||
| entry | conditions | time (min) | 35 | 36 | impurityc |
| 1 | 10% piperidine, 10% H2O in DMF | 30 | 0 | 100 | 0 |
| 2 | 0.6 M potassium silanolate in THF | 30 | 0 | 100 | 0 |
| 3 | 15% N2H4 in DMF | 75 | 9 | 91 | 0 |
| 4 | 15% N2H4 in DMF | 180 | 34 | 64 | 2 |
| 5 | 15% N2H4, 15% MeOH in THF | 300 | 100 | 0 | 0 |
| 6 | 15% N2H4, 15% MeOH in THF | 180 | 99 | >1 | 0 |
| 7 | 15% N2H4, 15% MeOH in THF | 75 | 78 | 22 | 0 |
| 8 | 15% N2H4, 15% MeOH in THF | 10 | 15 | 85 | 0 |
| 9 | 3% N2H4, 15% MeOH in THF | 75 | 3 | 97 | 0 |
| 10 | 15% 0.5 M LiOH, 15% MeOH in THF | 180 | 49 | 0 | 51 |
| 11 | 15% 0.5 M CsOH, 15% MeOH in THF | 180 | 40 | 0 | 60 |
NDP-α-MSH Rink resin 34 (30 μL of resin slurry in THF) was placed into a small fritted 2 mL syringe and washed with an N-TFA cleavage solvent mixture. After the treatment, the resin was washed with THF and CH2Cl2, and the peptide was cleaved off the resin and side-chain protecting groups removed as described in the Materials and Methods section. The peptide was then dissolved in 30% aqueous MeCN and analyzed by HPLC using a linear MeCN/0.1% CF3CO2H aqueous gradient (10% to 40% in 30 min) at a flow rate of 1.0 mL/min.
Yields were determined as ratios of peak areas at 280 nm on HPLC analyses; product 35 had a retention time of 16.1 min, and product 36 had a retention time of 24.2 min.
Unidentified impurities.
