Abstract
Obscure gastrointestinal bleeding (OGIB) is defined as bleeding of an unknown origin that persists or recurs after negative initial upper and lower endoscopies. Several techniques, such as endoscopy, arteriography, scintigraphy and barium radiology are helpful for recognizing the bleeding source; nevertheless, in about 5%-10% of cases the bleeding lesion cannot be determined. The development of videocapsule endoscopy (VCE) has permitted a direct visualization of the small intestine mucosa. We will analyze those techniques in more detail. The diagnostic yield of CE for OGIB varies from 38% to 93%, being in the higher range in those cases with obscure-overt bleeding.
Keywords: Capsule endoscopy, Bleeding, Small bowel, Obscure, Hemorrhage
INTRODUCTION
Obscure gastrointestinal bleeding (OGIB) is defined as bleeding of an unknown origin that persists or recurs after negative initial upper and lower endoscopies[1]. It can be subclassified as either obscure-occult, detected only by positive fecal occult blood tests (FOBT) and/or iron deficiency anemia (IDA), or obscure-overt with recurrent or persistent visible episodes of bleeding[2].
Approximately 5% of all gastrointestinal bleeding[3] are of obscure origin, and the most frequent causes are found among these: esophagitis, Cameron ulcers, Dieulafoy lesions, angiodysplasias, GAVE, portal hypertensive gastropathy, small bowel neoplasms, hemobilia, Meckel diverticulum, Crohn’s disease, medication-induced mucosal lesions and a few others[2].
Several techniques, such as endoscopy, arteriography, scintigraphy and barium radiology, are helpful for recognizing the bleeding source; nevertheless, in about 5%-10% of cases the bleeding lesion cannot be determined[4]. Before 2001, the study of OGIB was deficient as the small bowel could not be reviewed reliably in its whole, but the development of videocapsule endoscopy (VCE) has permitted a direct visualization of the small intestine mucosa.
DIAGNOSTIC TECHNIQUES
Capsule endoscopy (CE)
Capsule endoscopy (CE) is a disposable 26 mm × 11 mm plastic capsule (Figure 1) consisting of an optical dome, 4 light-emitting electrodes, a sensor, 2 batteries and a micro transmitter. It acquires and transmits 2 frames per second (until the battery expires after 7 h ± 1 h[5]) to a sensor array attached to the patient[2]. Image features include a 140-degree field of view, 1:8 magnification, 1 to 30 mm depth of field and a minimum size for detection of about 0.1 mm[5].
Figure 1.

Hand holding a small bowel PillCam.
It is passively propelled by peristalsis and it captures images of the entire length of the small intestine. The main limitations are the lack of air insufflation, the unavailability of rinsing, taking biopsies or/and treating lesions[2].
The incidence of capsule retention accounts of less than 1%[2] and is generally related to the presence of endoluminal narrowing. For that reason, and intending to predict the risk of retention, patients with high suspicion of bowel narrowing (history of NSAID’s intake, Crohn’s Disease, occlusive symptoms or ischemic bowel disease) should undergo capsule endoscopy exploration after performing other techniques such as CT scan, small bowel series or M2A patency capsule.
The diagnostic yield of CE for OGIB varies from 38% to 93%[5], being in the higher range in those cases with obscure-overt bleeding[3]. The ability to exclude bleeding lesions is between 82.6% and 100%[2]; nevertheless, in up to 35% of cases the capsule doesn’t reach the cecum, probably due to slow gastric transit[2]. The most commonly detected lesions in the small bowel suspicious of being responsible for bleeding, that can be found on CE, are: angioectasia (Figure 2A), fresh blood (Figure 2B), ulceration (Figure 2C), polypoid or tumoral lesions (Figure 2D) and varices[5], and it seems that there is no significant difference in the diagnostic yield of CE in obscure-overt and obscure-occult bleeding[5,6]. One of the difficulties while reading the CE is to determine what has to be considered a positive finding with clinical significance, and a consensus still has to be reached[3]. In general, nonspecific mucosal changes (red spots in Figure 3, white spots, etc) are not considered positive findings; while angioectasias, tumors (Figure 4), masses or mucosal breaks should be included as positive findings.
Figure 2.
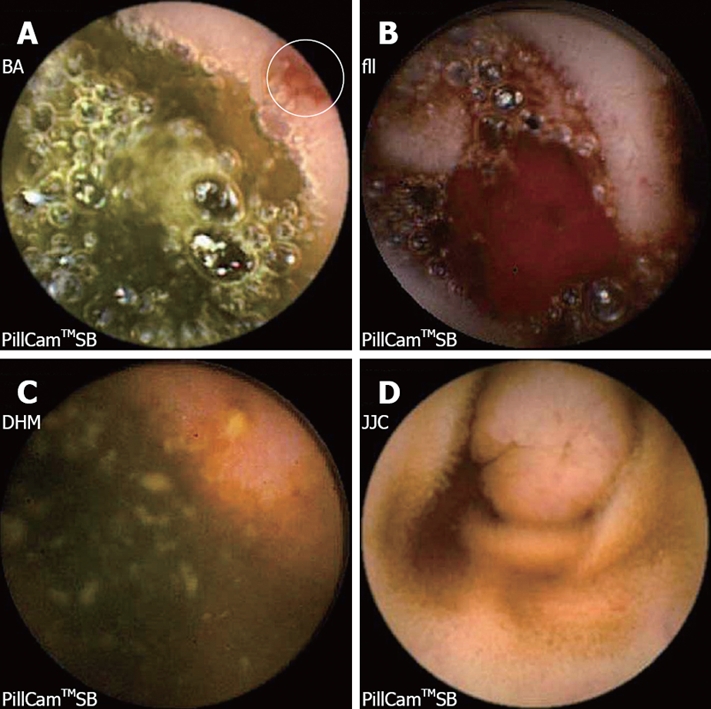
Lesions in the small bowel suspicious of being responsible for bleeding that can be found on CE: Angioectasia (A); Active bleeding (B); Ileal ulceration (C); Polypoid lesion (D).
Figure 3.
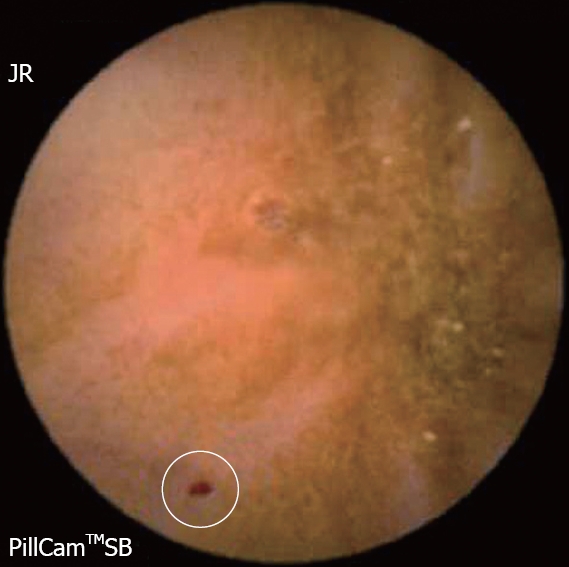
Red spot, with no relevant clinical significance.
Figure 4.
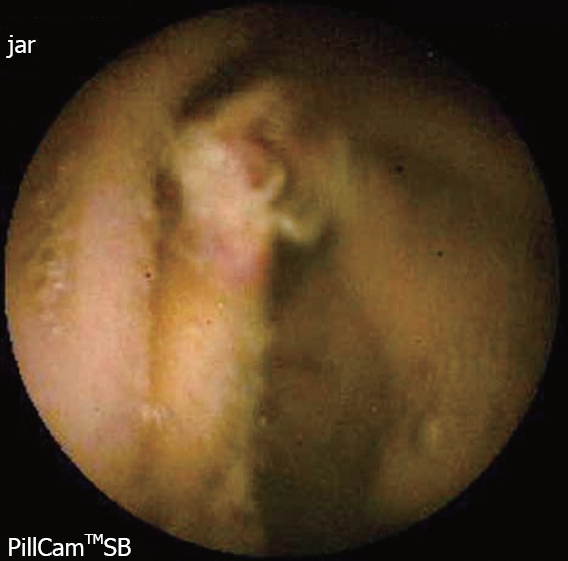
Ulcerated polyp.
This wide range of diagnostic yield can be related to several reasons, and one that has been suggested is the performance of CE within 15 days which can improve it (91% vs 34%, P < 0.001)[4]. Despite this improvement, some bleedings are still from an unknown origin. It has been suggested that a repeated CE can come across new findings in about 75% of cases, leading to changes in patient management in 62.5%[7] of cases. One of the main reasons to repeat a CE is the limited visualization that happens in about 44% of cases[7]. Recurrent bleeding is another reason for repeating CE. It can be helpful if the lesion responsible for bleeding is present intermittently or there was no bleeding source recognized in the first CE[7].
However, not only is it important to have a high diagnostic yield, but also to know if patient outcomes are improved after performing a CE. Carey et al considered measures of patient outcome the number of hospitalizations, units of blood transfused and the number of tests or procedures related to GI bleeding. Considering these measures, patient outcome appeared to improve after CE[3].
Lai et al[8] reported, in 2006, the results of a long-term follow-up of patients with obscure gastrointestinal bleeding. In 63.3% of cases, CE was able to determine the bleeding source and 32.7% of patients presented re-bleeding within the follow-up (median follow-up period: 19 mo)[8]. Patients with angiodysplasia were more susceptible to re-bleed (58.3% of cases were due to this condition), followed by patients with active bleeding during the CE procedure with no identified bleeding source (53.8%)[8]. They found that the probability of re-bleeding was significantly higher in patients with positive CE than in those with negative CE (P = 0.003)[8].
Repeat upper endoscopy and colonoscopy
Between 35% to 75% of patients can be under-diagnosed at the initial endoscopic study[2]. Missed lesions occur due to their size, location, presence of clots or the absence of active bleeding while the endoscopy is being performed. Other possible causes are anemia, volume contraction, the effect of sedatives, as they can result in paleness of vascular lesions, and the timing of the endoscopy, as it is more probably to identify the bleeding source if the endoscopy is performed within 48 hours of the acute event[2].
It has been recommended to repeat upper and lower endoscopy (“second-look endoscopy”)[2], nevertheless, about 5% to 10% of patients will remain undiagnosed.
Push enteroscopy
Enteroscopy consists of a peroral insertion into the jejunum of a long endoscope, using either a pediatric colonoscope or an enteroscope, and with or without the use of an overtube in order to avoid gastric looping[2]. This technique allows the examination of 15 to 160 cm beyond the ligament of Treitz[5] and it is generally considered the next diagnostic step after upper and lower negative endoscopic studies.
In 38%-75% of cases, the lesion causing OGIB can be identified with this technique[2,5]. However, in 28% to 75% of cases, it is reachable with a gastroscope[5]. Common findings are angiodysplasias (20%-46%), peptic ulcer disease, benign and malignant jejunal tumors, diverticulum, esophagitis and varices[2]. A meta-analysis has been published showing that the yield of CE for all findings is 63% vs 28% for Push Enteroscopy, with an incremental yield of 35%, P < 0.00001[9]. Yield of clinically significant findings is 56% for CE vs 26% for Push Enteroscopy[9].
Small bowel series and enteroclysis
Before the development of CE, a radiologic study of the small bowel was mandatory for the study of OGIB. The diagnostic yield is about 6%[2,5], and the most common missing lesions include angioectasia, ulcers and erosions[5]. The diagnostic yield can be improved (diagnostic yield of 10%-20%)[5] by performing enteroclysis. This technique consists in the introduction of a catheter into the small intestine followed by the injection of barium and methylcellulose. The barium coats the intestine and the methylcellulose distends the lumen to give a double contrast exam that allows for fluoroscopic visualization of the entire small bowel. The sensitivity of enteroclysis for small bowel tumors is higher than small bowel series[5].
A recent meta-analysis has shown that the diagnostic yield for all findings for CE is 67%, compared with an 8% for small bowel series. Diagnostic yield for significant findings is 42% for CE vs 6% for small bowel radiography[9].
Bleeding scanning with technetium-99-labeled RBC and angiography
This test can be useful if active bleeding is present as it may detect the source of hemorrhage if the bleeding rate ranges from 0.1 to 0.4 mL/min. Angiography can also be performed for diagnosis, as it is able to detect bleeding rates over 0.5 mL/min[2]. If the bleeding scanning is positive, an angiography should be performed to detect bleeding lesions and treat them if possible[2]. The diagnostic yield of these techniques varies from 44% to 68%[2].
Computed tomographic angiography (CTA)
Angiographic images can be obtained, not only by routine angiographic techniques, but also by CT scan. CTA is noninvasive and potentially useful for the diagnosis of GI bleeding, as it avoids the risks of standard angiography[10]. The CTA is able to identify the bleeding source in 24% of patients[10]. As has been recently published, CE is able to identify the bleeding source in a higher proportion of patients than CTA (72% vs 24%)[10].
Intraoperative enteroscopy (IE)
IE is the final diagnostic procedure for OGIB. It consists of performing an enteroscopy with the help of the surgeon, who helps by pushing the bowel over the enteroscope as the endoscopist examines it. The insertion can be done transoral or directly through a small enterotomy, but the last is more likely to achieve a complete examination of the small bowel. One of the drawbacks of this technique is that the manipulation of the bowel can create artifacts, which can be considered as bleeding sources although they are really not[2]. The direct effect of this handicap is that the examination of the mucosa has to be done while the intubation and not while the withdrawal of the endoscope. IE can achieve a diagnostic yield of a 70 to 93 which is comparable to CE[9].
Double balloon enteroscopy (DBE)
DBE is a novel endoscopic technique that allows for visualization of the entire small bowel, tissue sampling and therapeutic interventions. It consists of an enteroscope with 2 latex balloons, one attached to the endoscope and the other attached to an overtube. The procedure is performed by inflating and deflating the balloons, allowing the deep intubation through the small bowel. The route of insertion (perorally or transanally) selected depends on the patients’ symptoms (hematochezia, melena etc).
A potential bleeding cause can be found in 75.7% of patients[11] and approximately a quarter of patients might need both anterograde and retrograde approaches[11]. DBE can change patient management in about 83.5% of cases, with an average therapeutic endoscopic intervention of 15.7%[11]. Hadithi et al[12] have published that CE is able to detect the presence of a possible bleeding source in a higher proportion of patients than DBE (80% vs 60%).
CONCLUSION
As explained before, there are some diagnostic techniques that can be used for the study of OGIB. With so many different tools, it is important to establish which should be performed first, not only taking into account the diagnostic yield but also the cost effectiveness of each one. Finally the suggested algorithm for OGIB by the International Consensus on Capsule Endoscopy is shown in Figure 5.
Figure 5.
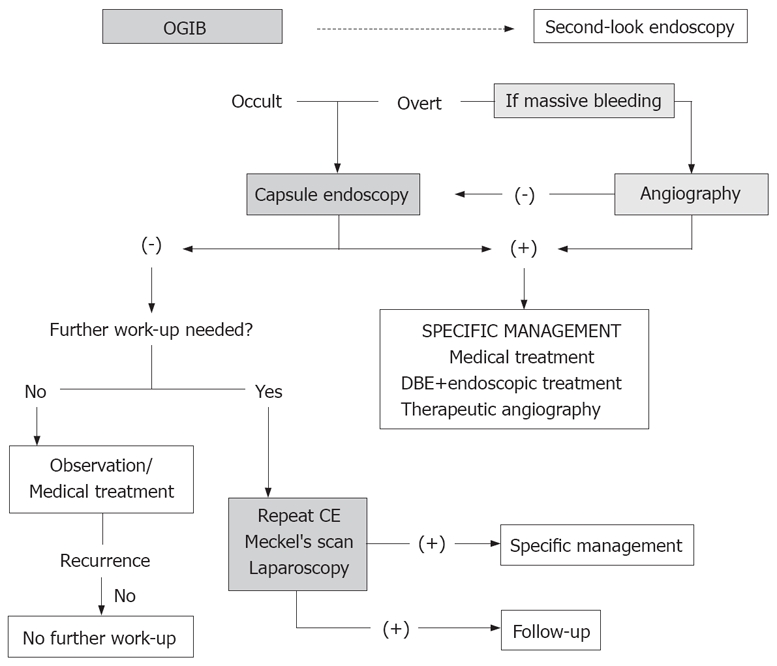
Suggested algorithm for OGIB.
Footnotes
Peer reviewer: Ronan A Cahill, Department of General Surgery, Waterford Regional Hospital, Waterford, Cork, Ireland
S- Editor Zhong XY L- Editor Rippe RA E- Editor Lin YP
References
- 1.Zuckerman GR, Prakash C, Askin MP, Lewis BS. AGA technical review on the evaluation and management of occult and obscure gastrointestinal bleeding. Gastroenterology. 2000;118:201–221. doi: 10.1016/s0016-5085(00)70430-6. [DOI] [PubMed] [Google Scholar]
- 2.Concha R, Amaro R, Barkin JS. Obscure gastrointestinal bleeding: diagnostic and therapeutic approach. J Clin Gastroenterol. 2007;41:242–251. doi: 10.1097/01.mcg.0000225616.79223.75. [DOI] [PubMed] [Google Scholar]
- 3.Carey EJ, Leighton JA, Heigh RI, Shiff AD, Sharma VK, Post JK, Fleischer DE. A single-center experience of 260 consecutive patients undergoing capsule endoscopy for obscure gastrointestinal bleeding. Am J Gastroenterol. 2007;102:89–95. doi: 10.1111/j.1572-0241.2006.00941.x. [DOI] [PubMed] [Google Scholar]
- 4.Bresci G, Parisi G, Bertoni M, Tumino E, Capria A. The role of video capsule endoscopy for evaluating obscure gastrointestinal bleeding: usefulness of early use. J Gastroenterol. 2005;40:256–259. doi: 10.1007/s00535-004-1532-5. [DOI] [PubMed] [Google Scholar]
- 5.Tang SJ, Haber GB. Capsule endoscopy in obscure gastrointestinal bleeding. Gastrointest Endosc Clin N Am. 2004;14:87–100. doi: 10.1016/j.giec.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Ben Soussan E, Antonietti M, Herve S, Savoye G, Ramirez S, Lecleire S, Ducrotte P, Lerebours E. Diagnostic yield and therapeutic implications of capsule endoscopy in obscure gastrointestinal bleeding. Gastroenterol Clin Biol. 2004;28:1068–1073. doi: 10.1016/s0399-8320(04)95183-4. [DOI] [PubMed] [Google Scholar]
- 7.Jones BH, Fleischer DE, Sharma VK, Heigh RI, Shiff AD, Hernandez JL, Leighton JA. Yield of repeat wireless video capsule endoscopy in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2005;100:1058–1064. doi: 10.1111/j.1572-0241.2005.40722.x. [DOI] [PubMed] [Google Scholar]
- 8.Lai LH, Wong GL, Chow DK, Lau JY, Sung JJ, Leung WK. Long-term follow-up of patients with obscure gastrointestinal bleeding after negative capsule endoscopy. Am J Gastroenterol. 2006;101:1224–1228. doi: 10.1111/j.1572-0241.2006.00565.x. [DOI] [PubMed] [Google Scholar]
- 9.Triester SL, Leighton JA, Leontiadis GI, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2005;100:2407–2418. doi: 10.1111/j.1572-0241.2005.00274.x. [DOI] [PubMed] [Google Scholar]
- 10.Saperas E, Dot J, Videla S, Alvarez-Castells A, Perez-Lafuente M, Armengol JR, Malagelada JR. Capsule endoscopy versus computed tomographic or standard angiography for the diagnosis of obscure gastrointestinal bleeding. Am J Gastroenterol. 2007;102:731–737. doi: 10.1111/j.1572-0241.2007.01058.x. [DOI] [PubMed] [Google Scholar]
- 11.Sun B, Rajan E, Cheng S, Shen R, Zhang C, Zhang S, Wu Y, Zhong J. Diagnostic yield and therapeutic impact of double-balloon enteroscopy in a large cohort of patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2006;101:2011–2015. doi: 10.1111/j.1572-0241.2006.00664.x. [DOI] [PubMed] [Google Scholar]
- 12.Hadithi M, Heine GD, Jacobs MA, van Bodegraven AA, Mulder CJ. A prospective study comparing video capsule endoscopy with double-balloon enteroscopy in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2006;101:52–57. doi: 10.1111/j.1572-0241.2005.00346.x. [DOI] [PubMed] [Google Scholar]


