Abstract
Small bowel strictures can be missed by current diagnostic methods. The Patency capsule is a new non-endoscopic dissolvable capsule which has as an objective of checking the patency of digestive tract, in a non-invasive manner. The available clinical trials have demonstrated that the Patency© capsule is a good tool for assessment of the functional patency of the small bowel, and it allows identification of those patients who can safely undergo a capsule endoscopy, despite clinical and radiographic evidence of small-bowel obstruction. Some cases of intestinal occlusion have been reported with the Patency© capsule, four of them needed surgery. So, a new capsule with two timer plugs (Agile© capsule) has been recently developed in order to minimize the risk of occlusion. This new device stars its dissolution process earlier (30 h after ingestion) and its two timer plugs have been designed to begin the disintegration even when the device is blocked in a tight stricture.
Keywords: Capsule endoscopy, Patency capsule, Agile capsule, Small bowel strictures
INTRODUCTION AND DEVICE DESCRIPTION
Since Iddan et al[1] reported the new wireless endoscopy system the capsule endoscopy (CE) has become one of the most significant technical innovations of Gastroenterology in recent years. Regarding the complications of the techniques, some incidental cases of impaction in a Meckel´s diverticulum[2], a Zenker´s diverticulum[3], or in the cricopharyngeal muscle[4], as well as aspiration of the capsule[5-7], solved without complications, have been reported. However, the most frequent side effect is undoubtedly the non-natural excretion (NNE) of the capsule due to a stricture or a tumor in the small bowel. The retrospective analysis of some series indicates that the incidence of NNE depends on the indication for the capsule exam: 0% in healthy controls[8], 1.4% in obscure gastrointestinal bleeding[9-13], 1.48% in suspected Crohn´s disease[14-17], 5%-13% in known Crohn´s disease[17-19] and 21% in suspected small bowel obstruction[20].
In the vast majority of cases, capsule retention, regardless of its cause, is asymptomatic, being evidenced by the absence of excretion and confirmed by means of radiology. On the other hand, capsule retention frequently allows for the identification of the bowel pathology which caused the symptoms in the patient and which could not have been diagnosed by means of standard methods, furthermore facilitating the location of the stricture or the tumor for the surgeon after “milking” the capsule through the bowel. For these reasons, many authors have described NNE as a “therapeutic complication” of the CE[21-23]. However, although this consideration is valid for patients with tumors or those with stricture due to NSAIDs, radiotherapy or previous abdominal surgery, it may not be valid in patients with stenoses secondary to Crohn’s disease. Unlike the previously mentioned groups, in patients with Crohn’s disease, surgery is not curative and may present more complications, thus in this case, NNE of the capsule can become more an “undesirable circumstance” than a “therapeutic complication”. The same can be said of those patients showing high surgical risk or who would not be willing to undergo surgery in the event that NNE occurs.
Current imaging techniques can show long or medium stenoses, with great reduction of the lumen size; however, short stenoses usually cannot be detected by standard methods. This fact explains that in most of the reported cases of non-natural excretion (NNE) of the capsule, the previous performance of the usual radiological studies was not capable of diagnosing the intestinal strictures which the capsule clearly showed[9,11-13,19-21]. It is, therefore, proven that the lack of findings in radiological techniques does not rule out the existence of a bowel stenosis.
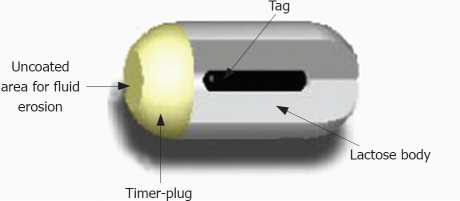
The manufacturing company of the PillCamSB has recently developed a new system (Given® M2A Patency System) which has as an objective of checking the patency of digestive tract, in a non-invasive manner. The Patency Capsule (PC) consists of a small identification tag (RFID), detectable by radiofrequency, which is surrounded by an absorbable material with a small amount of barium, all this covered by an external cover (Figure 1). PC has the same dimensions (11.4 mm × 26.4 mm) and the same shape as the standard capsule. PC is designed to remain intact in the gastrointestinal tract for about 80 h. After this period, if still within the body, it spontaneously disintegrates, except for the identification tag, whose small size (3 mm × 13 mm) allows it to pass through a stenosis of a very reduced lumen size. The persistence of the PC inside the organism can be verified by means of radiology, or with a radiofrequency emitting external detector device locating the identification tag.
Figure 1.
Schematic drawing of M2A® patency Capsule.
CLINICAL TRIALS WITH PATENCY CAPSULE
Clinical experience with PC is still limited. A prospective, multi-center trial was designed to assess the clinical usefulness and safety of the PC capsule in patients with intestinal strictures suspected from clinical and/or radiological data. The global data of this trial have not been published, but the results of four of these centers have been reported (Table 1)[24-27]. In the series reported by Spada et al[24], 30 out of 34 (88.2%) patients with known or suspected small bowel stricture retrieved the capsule in the stool. After the excretion, the PC was intact in 20 cases (median transit time 22 h), and disintegrated in 10 patients (median transit time 53 h). Ten patients underwent video capsule endoscopy following the patency capsule examination. In all of these the video capsule passed through the small-bowel stricture without complication. The rate of patients with the PC excreted intact was similar in the seria of Boivin et al[25] (16/22, 73%), Delvaux et al[26] (16/22, 73%), Signorelli et al[27] (26/32, 81%). In our center, the percentage of cases with PC excreted intact was significant lower (13/29, 45%), probably because of patient selection[28]. In all the seria, the patency system scanner showed a good agreement (94%-100%) with fluoroscopy findings in identifying the presence of the tag in the body and may be used to detect the presence of the patency capsule without the need for radiology.
Table 1.
Summary of the main seria on Patency Capsule (%)
| Author | Inclusion criteria | n | Diagnosis known or suspected before Pillcam | Capsule integrity at egestion | Pts with uneventful PillCam after PC excreted intact |
| Spada et al[24] 2005 | Suspected or confirmed small bowel stricture based on radiological exams | 34 | Crohn's disease: 30/34 (88.23) | 30/34 (88.23) | 10/10 (100) |
| Adhesional syndrome: 3/34 (8.82) | |||||
| Ischemic enteritis: 1/34 (2.94) | |||||
| Boivin et al[25] 2005 | Obstructive small bowel symptoms, and/or radiographic evidence of structuring small bowel disease | 22 | Crohn's disease: 15/22 (68.18) | 16/22 (72.73) | 13/13 (100) |
| Adhesional syndrome: 4/22 (18.18) | |||||
| Others: 3/22 (13.64) | |||||
| Delvaux et al[26] 2005 | Suspected or confirmed small bowel stricture based on either clinical background or radiological exams | 22 | Crohn's disease: 12/22 (54.54) | 16/22 (72.73) | 5/5 (100) |
| NSAIDs stricture: 3/22 (13.64) | |||||
| Tumors: 3/22 (13.64) | |||||
| Others: 4/22 (18.18) | |||||
| Signorelli et al[27] 2006 | Risk of capsule retention because of clinical background or radiological exams | 32 | Crohn's disease: 18/32 (56.25) | 26/32 (81.25) | 25/25 (100) |
| Intestinal surgery: 7/32 (21.87) | |||||
| Others: 7/32 (21.87) | |||||
| Caunedo et al[28] 2003 | Suspected or confirmed small bowel stricture based on radiological exams | 29 | Crohn's disease: 15/29 (51.72) | 13/29 (44.83) | 12/12 (100) |
| Adhesional syndrome: 6/29 (20.69) | |||||
| Tumors: 3/29 (10.34) | |||||
| Others: 5/29 (17.24) | |||||
| Spada et al[31] 2007 | Radiologic findings suggesting small bowel stricture without clinical evidence of obstruction | 27 | Crohn's disease: 24/27 (88.89) | 15/27 (55.55) | 15/15 (100) |
| Adhesional syndrome: 2/27 (7.41) | |||||
| Ischemic enteritis: 1/27 (3.70) | |||||
| Total | 166 | Crohn's disease: 114/166 (68.67) | 116/166 (69.88) | 80/80 (100) | |
| Adhesional syndrome: 15/166 (9.04) | |||||
| Tumors: 6/166 (3.61) | |||||
| Intestinal surgery: 7/166 (4.22) | |||||
| Others: 24/166 (14.46) |
All these authors conclude that PC was unable to detect the presence of a small bowel stricture as previously defined by radiological techniques, but it added crucial information on the functional patency of the stenoses, and this information could allow a distinction between rigid fibrotic strictures and flexible ones. Boivin et al[25] found that passage of an intact capsule that is accompanied by severe pain, similar to disintegration of the capsule with or without pain, seems indicative of a clinically relevant small-bowel stricture and is associated with a high probability of surgery. This observation seems to be confirmed by a recent retrospective analysis of 42 patients who underwent PC with known or suspected small bowel stricture[29]. In this study, the rate of patients who need to be operated in a period of three months was significantly higher in those with a delayed excretion, with the capsule excreted deformed, or with pain during the procedure. Moreover, in patients where painless natural expulsion of the intact PC occurred, CE could be applied without problems despite radiographic evidence of small-bowel strictures. This is an important finding since it might open the path to CE for about 60% of patients where video capsule investigation would otherwise be denied on grounds of history and radiological findings.
Abdominal pain during the procedure seems to be the main complication of PC (Table 2), observed in almost 20% of the cases reported to date. Probably, this pain is secondary to symptomatic intestinal occlusion, and it resolves spontaneously when the disintegration process concludes. However, at least four cases of occlusion did not respond adequately to conservative treatment and needed surgery. According to Gay et al[30], the problem could be that when the PC entrapped in a very tight stenosis it might not have been in contact with fluids and, therefore, only started to dissolve 48 h later, after moving back to the enlarged intestinal loop where it encountered fluids. Another possible factor is proposed by Gay et al[30]; PC is mainly made of lactose and the presence of lactase, an enzyme produced by intestinal mucosal cells, may be of importance in initiating the dissolution process. As lactase is mainly produced in the jejunum, one may also assume the enzyme is massively destroyed before the intestinal content reaches the ileum and that in the cases of occlusion, the enzyme could not have interacted with the capsule material. These authors[26,31] conclude that the start of dissolution at 40 h after ingestion is too slow to prevent episodes of intestinal occlusion, and so, it should be used cautiously under clinical surveillance in patients with Crohn´s disease.
Table 2.
Complications reported in the main seria on Patency Capsule (%)
| Author | Pts with abdominal pain during procedure | Severity of adverse event (abdominal pain) | Action taken |
| Spada et al[24] 2005 | 6/34 (17.64) | Mild: 5/34 (14.71) | Nothing: 5/34 (14.71) |
| Moderate: 0/34 (0) | Medical therapy: 1/34 (2.94) | ||
| Severe: 1/34 (2.94) | Surgery: 0/34 (0) | ||
| Boivin et al[25] 2005 | 6/22 (27.27) | Mild: 1/22 (4.54) | Nothing or medical therapy: 5/22 (22.73) |
| Moderate: 1/22 (4.54) | Surgery: 1/22 (4.54) | ||
| Severe: 4/22 (18.18) | |||
| Delvaux et al[26] 2005 | 3/22 (13.64) | Mild: 1/22 (4.54) | Nothing: 1/22 (4.54) |
| Moderate: 0/22 (0) | Medical therapy: 0/22 (0) | ||
| Severe: 2/22 (9.09) | Surgery: 2/22 (9.09) | ||
| Signorelli et al[27] 2006 | 2/32 (6.25) | Mild: 2/32 (1.44) | Nothing: 2/32 (1.44) |
| Moderate: 0/32 (0) | Medical therapy: 0/32 (0) | ||
| Severe: 0/32 (0) | Surgery: 0/32 (0) | ||
| Caunedo et al[28] 2003 | 10/29 (34.48) | Mild: 4/29 (13.79) | Nothing: 3/29 (10.35) |
| Moderate: 4/29 (13.79) | Medical therapy: 7/29 (24.14) | ||
| Severe: 2/29 (6.90) | Surgery: 0/29 (0) | ||
| Spada et al[31] 2007 | 6/27 (22.22) | Mild: 5/27 (18.52) | Nothing or medical therapy: 5/27 (18.52) |
| Moderate: 0/27 (0) | Surgery: 1/27 (3.70) | ||
| Severe: 1/27 (3.70) | |||
| Total | 33/166 (19.88) | Mild: 18/166 (10.84) | Nothing or medical therapy: 29/166 (17.47) |
| Moderate: 5/166 (3.01) | Surgery: 4/166 (2.41) | ||
| Severe: 10/166 (6.02) |
THE AGILE© PATENCY CAPSULE
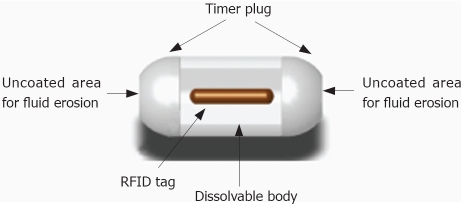
In order to reduce the risk of obstruction, a new dissolvable capsule with two timer plugs, one at each end, has been recently developed (Figure 2). With these two timer plugs, the dissolution process starts earlier (30 h), and the contact with intestinal fluids is ensured even if the device is blocked in a tight stricture[32]. The new capsule, named Agile© Patency Capsule, has been evaluated in a multicenter clinical trial[33] designed to assess its safety in patients with known strictures and its ability to help physicians identify which patients may safely undergo CE. In this study, the intestinal tract was considered to be sufficiently patent if the capsule was excreted intact, or if the capsule was not detected by the scanner at 30 h after ingestion. If patency was established, then the patient underwent CE. Fifty-nine out (56%) of the 106 included patients excreted the Agile© capsule intact and subsequently underwent CE. There were no cases of retention of the video capsule and no Agile© capsules were found to have dissolved before 30 h after ingestion. Significant findings on CE were found in 24 patients (41%). A total of 17 (17/106) subjects had an adverse event, of these, 11 (11/106) consisted of abdominal pain. The pain resolved with conservative management within 48 h in all except for a patient with Crohn´s disease who need surgery (1/106). This patient developed obstruction after ingestion of the AGILE capsule and underwent surgery with resection of the terminal ileum and proximal colon, no remnants of the capsule were found at surgery. The physicians involved felt that the capsule did not lead to the obstruction. All of the other adverse events resolved within 48 h with conservative management.
Figure 2.
Schematic drawing of AGILE® patency Capsule.
In summary, the AGILE Patency capsule seems to be a useful, non-invasive tool to identify which patients with suspected strictures could safely ingest the standard video capsule. It has been designed to minimize the risk of intestinal occlusion.
Footnotes
Peer reviewer: Amado S Peña, Professor, Department of Pathology, Immunogenetics, VU University Medical Centre, De Boelelaan 1117, PO Box 7057, Amsterdam 1007 MB, The Netherlands
S- Editor Zhong XY L- Editor Rippe RA E- Editor Lin YP
References
- 1.Iddan G, Meron G, Glukhovsky A, Swain P. Wireless capsule endoscopy. Nature. 2000;405:417. doi: 10.1038/35013140. [DOI] [PubMed] [Google Scholar]
- 2.Gortzak Y, Lantsberg L, Odes HS. Video Capsule entrapped in a Meckel's diverticulum. J Clin Gastroenterol. 2003;37:270–271. doi: 10.1097/00004836-200309000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Feitoza AB, Gostout CJ, Knipschield MA, Rajan E. Video capsule endoscopy: Is the recording time ideal? Am J Gastroenterology. 2002;97:S307. [Google Scholar]
- 4.Fleischer DE, Heigh RI, Nguyen CC, Leighton JA, Sharma VK, Musil D. Videocapsule impaction at the cricopharyngeus: a first report of this complication and its successful resolution. Gastrointest Endosc. 2003;57:427–428. doi: 10.1067/mge.2003.118. [DOI] [PubMed] [Google Scholar]
- 5.Schneider AR, Hoepffner N, Rosch W, Caspary WF. Aspiration of an M2A capsule. Endoscopy. 2003;35:713. doi: 10.1055/s-2003-41527. [DOI] [PubMed] [Google Scholar]
- 6.Sinn I, Neef B, Andus T. Aspiration of a capsule endoscope. Gastrointest Endosc. 2004;59:926–927. doi: 10.1016/s0016-5107(04)00291-3. [DOI] [PubMed] [Google Scholar]
- 7.Buchkremer F, Herrmann T, Stremmel W. Mild respiratory distress after wireless capsule endoscopy. Gut. 2004;53:472. doi: 10.1136/gut.2003.033845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein JL, Eisen GM, Lewis B, Gralnek IM, Zlotnick S, Fort JG. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol. 2005;3:133–141. doi: 10.1016/s1542-3565(04)00619-6. [DOI] [PubMed] [Google Scholar]
- 9.Barkin JS, Friedman S. Wireless capsule endoscopy requiring surgical intervention: The world’s experience. Am J Gastroenterol. 2002;97:A83. [Google Scholar]
- 10.Pennazio M, Santucci R, Rondonotti E, Abbiati C, Beccari G, Rossini FP, De Franchis R. Outcome of patients with obscure gastrointestinal bleeding after capsule endoscopy: report of 100 consecutive cases. Gastroenterology. 2004;126:643–653. doi: 10.1053/j.gastro.2003.11.057. [DOI] [PubMed] [Google Scholar]
- 11.Sears DM, Avots-Avotins A, Culp K, Gavin MW. Frequency and clinical outcome of capsule retention during capsule endoscopy for GI bleeding of obscure origin. Gastrointest Endosc. 2004;60:822–827. doi: 10.1016/s0016-5107(04)02019-x. [DOI] [PubMed] [Google Scholar]
- 12.Rondonotti E, Herrerias JM, Pennazio M, Caunedo A, Mascarenhas-Saraiva M, de Franchis R. Complications, limitations, and failures of capsule endoscopy: a review of 733 cases. Gastrointest Endosc. 2005;62:712–716; quiz 752, 754. doi: 10.1016/j.gie.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Caunedo A, Rodriguez-Tellez M, Garcia-Montes JM, Gomez-Rodriguez BJ, Guerrero J, Herrerias JM Jr, Pellicer F, Herrerias JM. Usefulness of capsule endoscopy in patients with suspected small bowel disease. Rev Esp Enferm Dig. 2004;96:10–21. doi: 10.4321/s1130-01082004000100003. [DOI] [PubMed] [Google Scholar]
- 14.Fireman Z, Mahajna E, Broide E, Shapiro M, Fich L, Sternberg A, Kopelman Y, Scapa E. Diagnosing small bowel Crohn's disease with wireless capsule endoscopy. Gut. 2003;52:390–392. doi: 10.1136/gut.52.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eliakim R, Fischer D, Suissa A, Yassin K, Katz D, Guttman N, Migdal M. Wireless capsule video endoscopy is a superior diagnostic tool in comparison to barium follow-through and computerized tomography in patients with suspected Crohn's disease. Eur J Gastroenterol Hepatol. 2003;15:363–367. doi: 10.1097/00042737-200304000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Herrerias JM, Caunedo A, Rodriguez-Tellez M, Pellicer F, Herrerias JM Jr. Capsule endoscopy in patients with suspected Crohn's disease and negative endoscopy. Endoscopy. 2003;35:564–568. doi: 10.1055/s-2003-40241. [DOI] [PubMed] [Google Scholar]
- 17.Cheifetz AS, Kornbluth AA, Legnani P, Schmelkin I, Brown A, Lichtiger S, Lewis BS. The risk of retention of the capsule endoscope in patients with known or suspected Crohn's disease. Am J Gastroenterol. 2006;101:2218–2222. doi: 10.1111/j.1572-0241.2006.00761.x. [DOI] [PubMed] [Google Scholar]
- 18.Mow WS, Lo SK, Targan SR, Dubinsky MC, Treyzon L, Abreu-Martin MT, Papadakis KA, Vasiliauskas EA. Initial experience with wireless capsule enteroscopy in the diagnosis and management of inflammatory bowel disease. Clin Gastroenterol Hepatol. 2004;2:31–40. doi: 10.1016/s1542-3565(03)00289-1. [DOI] [PubMed] [Google Scholar]
- 19.Buchman AL, Miller FH, Wallin A, Chowdhry AA, Ahn C. Videocapsule endoscopy versus barium contrast studies for the diagnosis of Crohn's disease recurrence involving the small intestine. Am J Gastroenterol. 2004;99:2171–2177. doi: 10.1111/j.1572-0241.2004.40253.x. [DOI] [PubMed] [Google Scholar]
- 20.Cheifetz AS, Lewis BS. Capsule endoscopy retention: is it a complication? J Clin Gastroenterol. 2006;40:688–691. doi: 10.1097/00004836-200609000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Cave D, Legnani P, de Franchis R, Lewis BS. ICCE consensus for capsule retention. Endoscopy. 2005;37:1065–1067. doi: 10.1055/s-2005-870264. [DOI] [PubMed] [Google Scholar]
- 22.Brandt LJ. Deformation of the anus: an alternative to rectal air suctioning for patient comfort after colonoscopy. Gastrointest Endosc. 2004;59:461. doi: 10.1016/s0016-5107(03)02373-3. [DOI] [PubMed] [Google Scholar]
- 23.Baichi MM, Arifuddin RM, Mantry PS. What we have learned from 5 cases of permanent capsule retention. Gastrointest Endosc. 2006;64:283–287. doi: 10.1016/j.gie.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 24.Spada C, Spera G, Riccioni M, Biancone L, Petruzziello L, Tringali A, Familiari P, Marchese M, Onder G, Mutignani M, et al. A novel diagnostic tool for detecting functional patency of the small bowel: the Given patency capsule. Endoscopy. 2005;37:793–800. doi: 10.1055/s-2005-870246. [DOI] [PubMed] [Google Scholar]
- 25.Boivin ML, Lochs H, Voderholzer WA. Does passage of a patency capsule indicate small-bowel patency? A prospective clinical trial? Endoscopy. 2005;37:808–815. doi: 10.1055/s-2005-870220. [DOI] [PubMed] [Google Scholar]
- 26.Delvaux M, Ben Soussan E, Laurent V, Lerebours E, Gay G. Clinical evaluation of the use of the M2A patency capsule system before a capsule endoscopy procedure, in patients with known or suspected intestinal stenosis. Endoscopy. 2005;37:801–807. doi: 10.1055/s-2005-870241. [DOI] [PubMed] [Google Scholar]
- 27.Signorelli C, Rondonotti E, Villa F, Abbiati C, Beccari G, Avesani EC, Vecchi M, de Franchis R. Use of the Given Patency System for the screening of patients at high risk for capsule retention. Dig Liver Dis. 2006;38:326–330. doi: 10.1016/j.dld.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Caunedo A, Rodriguez-Tellez M, Romero J, Hernandez-Duran M, Romero R, Pellicer FJ, Herrerias JM. Evaluation of the M2A Patency capsule in the gastrointestinal tract: one-centre preliminary data from a multicentre prospective trial. Endoscopy. 2003;35:A182. [Google Scholar]
- 29.Caunedo-Alvarez A, Romero-Vázquez J, Gomez-Rodriguez BJ, Sanchez-Yague A, Castro-Laria L, Herrerias-Gutierrez JM. Prognostic Factors of Short-Term Surgery in Patients with Known or Suspected Stricture Undergone to Patency Capsule. Gastrointes Endoscopy. 2007;65:AB340. [Google Scholar]
- 30.Gay G, Delvaux M, Laurent V, Reibel N, Regent D, Grosdidier G, Roche JF. Temporary intestinal occlusion induced by a "patency capsule" in a patient with Crohn's disease. Endoscopy. 2005;37:174–177. doi: 10.1055/s-2004-826195. [DOI] [PubMed] [Google Scholar]
- 31.Spada C, Shah SK, Riccioni ME, Spera G, Marchese M, Iacopini F, Familiari P, Costamagna G. Video capsule endoscopy in patients with known or suspected small bowel stricture previously tested with the dissolving patency capsule. J Clin Gastroenterol. 2007;41:576–582. doi: 10.1097/01.mcg.0000225633.14663.64. [DOI] [PubMed] [Google Scholar]
- 32.Caunedo-Alvarez A, Romero-Vazquez J, Gomez-Rodriguez BJ, Sanchez-Yague A, Castro-Laria L, Herrerias-Gutierrez JM. Evaluation of a new double-headed biodegradable device (AGILE Patency Capsule) for detecting functional patency of the small intestine: A prospective clinical trial. Proceedings of the 5th International Conference on Capsule. Endoscopy. 2006 [Google Scholar]
- 33.Herrerias JM, Leighton JA, Costamagna G, Infantolino A, Eliakim R, Fischer D, Rubin DT, Manten HD, Scapa E, Morgan DR, et al. Agile patency system eliminates risk of capsule retention in patients with known intestinal strictures who undergo capsule endoscopy. Gastrointest Endosc. 2008;67:902–909. doi: 10.1016/j.gie.2007.10.063. [DOI] [PubMed] [Google Scholar]