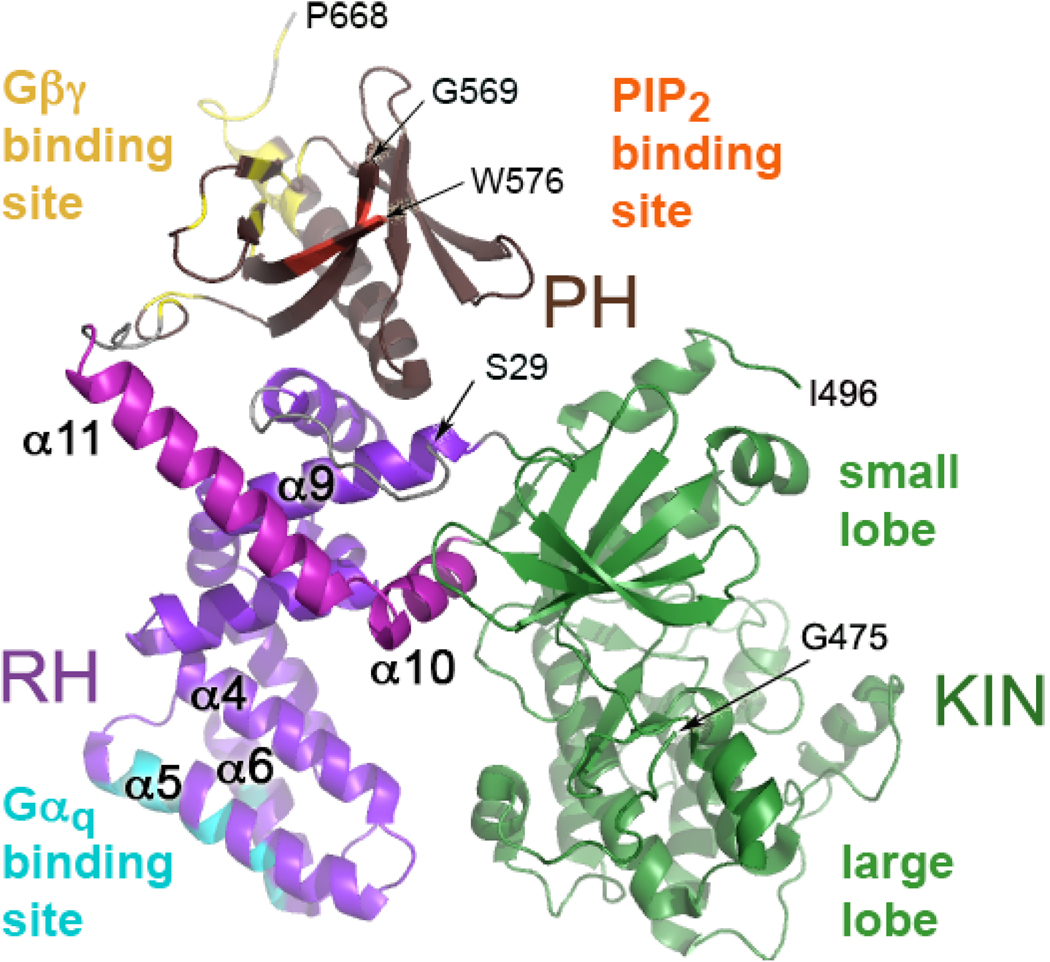
Figure 1. GRK2 Structure.
Full-length GRK2 is oriented to display its predicted membrane-proximal surface, regions of which are expected to interact with the cytoplasmic loops and tails of an activated GPCR (6). In GRK2, the RH domain contacts both the kinase domain (green) and the PH domain (brown). In the RH domain, α-helices 1–9 are purple while α-helices 10 and 11, which are unique to the RH domains of GRKs, are magenta. The regions that interact with Gαq are colored cyan, that interact with Gβγ are yellow, and that interact with phosphatidylinositol bisphosphate (PIP2) are orange. The N-terminal 28 amino acids, a large portion of the kinase extension (476–495), a loop of the PH domain, and the C-terminal 21 residues are not structured in GRK2 crystals. The termini of ordered regions are indicated by Ser29, Gly475, Ile496, Gly569, Trp576, and Pro668 (arrows).