Abstract
Transforming growth factor beta 1 (TGF-β1) levels are increased in the peritoneal fluid of endometriosis patients, and endometrial cells express TGF-β signaling components; however, little is known regarding the role of TGF-β in endometriosis. Our objective was to examine the effects of TGF-β1 on (i) the expression of macrophage colony-stimulating factor receptor encoded by the c-fms gene, (ii) transmesothelial invasiveness of endometrial cells, (iii) cellular proliferation and (iv) attachment to peritoneal mesothelial cells (PMCs). Effects of TGF-β1 on c-fms mRNA expression were determined by real-time RT–PCR and c-fms cell-surface expression by flow cytometry. Effects of TGF-β1 on the invasiveness of the immortalized endometrial epithelial cell (EEC) line EM42 and primary EECs were examined using a three-dimensional in vitro system modeling the peritoneum. Cellular proliferation and attachment to PMCs were also examined using established techniques. TGF-β1 had little or no effect on cellular proliferation and endometrial cell attachment to PMCs. TGF-β1 significantly induced the expression of c-fms mRNA and c-fms cell-surface expression. TGF-β1 enhanced transmesothelial invasion by EM42 cells and EECs. Antagonists of TGF-β1 signaling significantly inhibited both the induction of c-fms expression and cellular invasiveness, suggesting that additional studies are warranted to assess the therapeutic potential of TGF-β antagonists in endometriosis.
Keywords: transforming growth factor beta, c-fms, endometriosis, transmesothelial invasion, TGFBR 1 inhibitors
Introduction
Endometriosis, a benign gynecological disorder characterized by the ectopic growth of endometrial tissue, is commonly associated with infertility and pelvic pain (Eskenazi and Warner, 1997). Although the etiology of endometriosis is not fully understood, a widely accepted hypothesis proposes that retrograde menstruation through the Fallopian tube is a means for the transfer of endometrial tissue into the peritoneal cavity, where it implants on pelvic structures. The establishment of endometriotic lesions in the peritoneal cavity requires adhesion, invasion and proliferation of the ectopic endometrial tissue. Although endometriosis is usually a benign condition, the invasiveness and growth of endometrial tissue in endometriosis resemble those of invasive carcinomas in some respects. Studies have shown that cytokines and growth factors active in carcinogenesis may also play relevant roles in endometriosis (Vassiliadis et al., 2005).
Transforming growth factor beta (TGF-β) is a pleiotropic factor involved in cellular differentiation and tissue remodeling in several organs, including the endometrium (Jones et al., 2006). The three TGF-β isoforms (numbered 1–3) are differentially expressed in the endometrium, with TGF-β 1 and 3 expressed in epithelial and stromal cells and TGF-β2 expressed mainly in the stroma (Jones et al., 2006). TGF-β strongly inhibits cellular proliferation, with tumor-suppressor functions in several malignancies (Jakowlew, 2006). During malignant progression, TGF-β mediates pro-carcinogenic effects including cellular invasiveness, angiogenesis, and immune suppression (Jakowlew, 2006; Wrzesinski et al., 2007). TGF-β signals via cell-surface receptors TGFBR I and TGFBR II and the proteoglycan TGFBR III (Heldin et al., 1997; Dennler et al., 2002). TGFBR II and III are the ligand-binding receptors. Upon stimulation by TGF-β, TGFBR II and TGFBR III interaction with TGFBR I leads to the activation of the intracellular Smad signaling system that includes Smad2 and Smad3 (Dennler et al., 2002). When activated, these Smads bind to the co-Smad Smad4, leading to their nuclear transport and transactivation of TGF-β-responsive genes via Smad-binding elements. An alternative signaling cascade involves the TGF-β-activated kinase, which activates the p38 MAP kinase and c-Jun N-terminal kinase pathways (Wrzesinski et al., 2007).
Published data have suggested that TGF-β may play a role in the etiology of endometriosis. Peritoneal fluid from endometriosis patients contains approximately 10-fold higher TGF-β levels when compared with that from endometriosis-free women (Oosterlynck et al., 1994), and TGF-β is increased in the stroma of endometriotic tissue samples (Loverro et al., 2001). Recently, Komiyama (2007) suggested that activated TGF-β1 levels are increased in endometriotic cysts compared with normal endometrium, an increase primarily due to enhanced epithelial cell expression. Smad signaling was detected in endometrial cells. Activated Smad 3 and Smad 4 were expressed in both endometrial stromal cells (ESCs) and epithelial cells (EECs), suggesting that these cell are responsive to TGF-β signaling (Luo et al., 2003a). Gonadotrophin-releasing hormone agonists (GnRHa), which are used in the treatment of endometriosis, increased the expression of the inhibitory Smad7 in the EEC line HES and decreased activated Smad3 levels, suggesting that the inhibitory effects on TGF-β signaling may mediate the therapeutic actions of GnRHa (Luo et al., 2003b).
We previously showed that TGF-β1 induced the expression of the macrophage colony-stimulating factor (CSF-1) receptor, encoded by the c-fms gene, in cervical cancer cells, suggesting a cross-talk between TGF-β1 and CSF-1 signaling (Kirma et al., 2007). CSF-1/c-fms signaling has been shown to be increased in both endometrial and breast cancers (Baiocchi et al., 1991). We previously showed that CSF-1 may play a role in endometrial cell invasion in vitro and in vivo (Jensen et al., 2008). We also showed that autocrine stimulation by CSF-1 enhances the growth of EM42 cells, an immortalized EEC line (Gill et al., 2001). Other evidence suggesting a role for CSF-1 in endometriosis includes the presence of its receptor c-fms in peritoneal mesothelial cells (PMCs) as well as endometrial stromal and epithelial cells (Wanichkul et al., 2003). Others have also shown that c-fms expression in endometriotic implants is increased when compared with eutopic endometrium (Mettler et al., 2004).
The regulation of the c-fms gene and the modulation of CSF-1 signaling have not been extensively studied in endometriosis. The c-fms gene is controlled by two promoters: the first is the trophoblast-specific promoter (promoter 1), initiating transcription at exon 1, and the second is the monocyte-specific promoter (promoter 2), initiating transcription at exon 2 (Roberts et al., 1992; Sapi et al., 1995; Hume et al., 1997). Thus, transcripts initiated by the trophoblast promoter contain exon 1, which is absent from the transcripts initiated by the macrophage-specific promoter. Both c-fms transcripts result in the same protein product, as the translational start site is present in exon 2 (Roberts et al., 1992; Sapi et al., 1995; Hume et al., 1997).
Our objective was to study the role of TGF-β1 in endometriosis. We have investigated whether TGF-β plays a role in the invasiveness of EECs, as well as cellular proliferation and attachment to PMCs. We have also examined whether TGF-β1 regulates c-fms expression in endometrial cells. The findings suggest that novel strategies in the treatment of endometriosis may involve targeting TGF-β signaling involved in cellular invasion, including components that may regulate c-fms as a means of modulating CSF-1 signaling in this disease.
Materials and Methods
Cell culture
EM42-immortalized endometrial cells were cultured in RPMI containing 10% fetal bovine serum (FBS) (Invitrogen, Carsbald, CA, USA) supplemented with glutamine. Eutopic EECs from proliferative phase endometrium were obtained from an IRB-approved endometriosis tissue repository in the Department of Obstetrics and Gynecology at the University of Texas Health Science Center at San Antonio. Primary EEC from the proliferative phase were purified and maintained in culture as described previously (Ferreira et al., 2008). EECs were grown in monolayer cultures in an enriched medium containing (volumes per liter of solution): mCDB 131 (Sigma–Aldrich, St Louis, MO, USA, 330 ml), Medium 199 (Sigma, 335 ml), Minimal Essential Medium—alpha modification (JRH Biosciences, Lenexa, KS, USA, 222 ml), antibiotics and antimycotics (10 ml), 10 µg/ml insulin (1 ml), d-glucose 0.3 µg/ml (667 µl) and FBS (100 ml). To eliminate the effects of steroid hormones and growth factors present in FBS in the media, cells were washed with phenol red-free Hanks buffer salt solution (Invitrogen) and incubated in phenol red-free RPMI media containing charcoal-stripped (C-S), heat-inactivated (H-I) FBS (10%) for 24–48 h before treatment. The media were then replaced with the same (containing 10% C-S, H-I FBS) and treatments added. Where appropriate, inhibitors were added at the same time as TGF-β1. Conditions of treatment (concentrations and duration) with TGF-β1 and antagonists are described later. Appropriate vehicle was added for control treatments. TGF-β1 and p38 inhibitor SB203580 were purchased from R&D Systems (Minneapolis, MN, USA) and Alexis Biochemicals (San Diego, CA, USA), respectively. SB431542 and A 83-01 were purchased from Sigma-Aldrich and Tocris (Elisville, MO, USA), respectively.
RNA expression studies
RNA was isolated using the Qiagen RNeasy plus (Valencia, CA, USA) with a genomic DNA removal step as per manufacturer's protocol. Reverse transcription (RT) was carried out using the Applied Biosystems kit (Foster City, CA, USA). Real-time PCR and subsequent analyses were carried out using Smartmix PCR beads (Cepheid, Sunnyvale, CA, USA) with 0.25× SybrGreen in the Cepheid SmartCycler to detect c-fms and the housekeeping gene GAPDH transcripts, as described previously (Kirma et al., 2007). The initial c-fms PCR primer set used in this study [termed herein as c-fms (internal) primer set], spanning exons 18 to 22, detects c-fms RNA transcripts initiated at both promoters 1 and 2. The c-fms (internal) and GAPDH primer set sequences were published previously (Kirma et al., 2007). The c-fms primers detecting transcripts containing exon 1 [termed herein as c-fms (exon 1/2) primer set] were designed corresponding to sequences in exon 1 (sense sequence) and exon 2 (antisense sequence): 5′-CAGAGTGTCCAAAAGCGTGA-3′ and 5′-GGACACACGTTCCTCTCCTC-3′, respectively. Transcripts initiated from c-fms promoter 1, but not promoter 2, are detected by this primer set. Melt curve analysis was performed after each real-time PCR to ascertain PCR product specificity. PCR reactions using c-fms (internal), c-fms (exon 1/2) and GAPDH primer sets gave unique melt peaks, indicative of discrete amplification products, at 87.7, 85.4 and 90.2°C, respectively. Real-time PCR assays were performed in duplicates and repeated at least three times. To confirm the role of TGF-β in the induction of c-fms expression, EM42 cells were treated with TGF-β1 (10 ng/ml) in the presence or absence of two potent TGFBR I antagonists SB431542 (10 µmol/l) and A 83-01 (5 µmol/l), and the p38 mitogen-activated protein kinase (MAPK) inhibitor SB203580 (10 µmol/l), for 16 h.
Flow cytometry
After treatment with TGF-β1 for 48 h to allow for protein synthesis, cells were stained with fluoroscein-conjugated anti-human c-fms (CD115) antibody (R&D Systems) according to the following procedure. Cells (1 × 105) were suspended in the phosphate-buffered saline (PBS) supplemented with 0.5% bovine serum albumin (BSA). Cells were FC-blocked with 1 µg of mouse IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 15 min, followed by incubation with the c-fms antibody for 45 min. Cells were washed with PBS (0.5% BSA) three times to eliminate unbound antibody and suspended in PBS (containing 1% paraformaldehyde). Cells were then subjected to detection by flow cytometry.
Chromatin immunoprecipitation assay
The activation of promoter 1 by TGF-β1 was investigated using the chromatin immunoprecipitation (ChIP) assay to detect the levels of chromatin-associated acetylated histone 3 (Ac-H3). ChIP assay was performed using the Active Motif (Carlsbad, CA, USA) ChIP-IT™ Express kit according to the manufacturer's directions. Briefly, EM42 cells were treated with TGF-β1 (10 ng/ml) for 15 and 30 min, which is sufficient time for signal translocation into the nucleus and promoter activation. Control was treated with vehicle. After indicated treatment times, cells were fixed with formaldehyde for 15 min, followed by addition of glycine. Cells were then washed with 1× PBS and lysed. Chromatin was sheered by sonication according to previously optimized conditions to about 600 bp fragments. A portion of the chromatin fragments was kept aside at −20°C to subsequently determine unprecipitated total chromatin (input) for normalization. Immunoprecipitation of chromatin fragments was carried out using anti-Ac-H3 rabbit IgG (Millipore/Upstate, Lake Placid, NY, USA) in the presence of protein G conjugated to magnetic beads. As a negative control for non-specific immunopreciptation, normal rabbit IgG (Millipore/Upstate) was used. Immunoprecipitation was performed overnight at 4°C. After washing the beads, the immunoprecipitated chromatin fragments were eluted, reverse-cross-linked and treated with proteinase K for 1 h at 37°C to eliminate proteins. The chromatin fragments were then purified using the Qiagen Qiaquick PCR purification kit. Specific primers were used to detect immunoprecipitated chromatin fragments, as well as input chromatin, containing the promoter 1 region at 339 bp upstream of exon 1 by real-time PCR (product size: 215 base pairs). Primer sequences: 5′-AAAGTGGGGATTTTCGCT-3′ (sense) and 5′-CTGATAGCCAAAGCCAAAGC-3′ (antisense). Unique melt peaks subsequent to PCR, indicative of discrete amplification products, were detected at 89°C. ChIP assays were repeated at least three times.
Cellular invasion assay
The 3D invasion assay modeling transmesothelial invasion was described previously (Ferreira et al., 2008; Nair et al., 2008). Briefly, 20 000 control and TGF β-treated cells (in the presence or absence of inhibitors) were plated after labeling with CellTracker Green® (Molecular Probes-Invitrogen) on a confluent layer of LP9 PMCs on top of growth factor-reduced Matrigel™, coated on 8-µm pore membranes in 24-well invasion chambers (BD Bioscience, San Jose, CA, USA). The invasion chambers were incubated at 37°C in 5% CO2 for 24 h. Cells that did not invade through the PMC layer and the matrigel, those on the upper surface of the membranes, were mechanically removed with cotton tip applicators. Invaded cells on the bottom of the coated membranes were visualized using a fluorescence microscope with a ×20 objective. Images were obtained from four standardized, non-overlapping fields covering ∼86% of the membrane. Invaded cells were counted using the Image J software (http://rsbweb.nih.gov/ij/). Invasion assays were performed in duplicates and repeated at least three times.
Cellular proliferation assay
Cellular proliferation was assayed using the CellTiter-Glo luminescence-based kit (Promega, Madison, WI, USA) according to the manufacturer's directions. Briefly, endometrial cells were plated in 96-well plates at 2000 cells/well for EM42 cells and EECs. After washing with PBS and incubation in DMEM media containing heat inactivated, C-S serum (10%) for 24 h, the cells were treated with TGF-β1 at indicated concentrations for 48 h. The CellTiter-Glo substrate was added to the cells to allow for the production of luminescence signal. Luminescence of a minimum of six replicates per treatment condition was measured in a Fluoroscan Ascent FL luminometer (Thermo Labsystems Waltham, MA, USA).
Attachment assay
Attachment assay of endometrial cells to mesothelial cells was performed as described previously (Nair et al., 2008). In brief, EM42 cells and EECs were labeled with calcein-AM (Molecular Probes; 5 µM) for 20 min at 37°C. The cells were plated at 20 000 cells per well over 96-well plates with confluent LP9 PMCs. After incubation at 37°C for 1 h in 5% CO2 in air, the plates were inverted, submerged in a bath of PBS containing calcium and magnesium (Invitrogen) and incubated at 37°C in 5% CO2 in air for 15 min on an orbital mixer (Barnstead/Thermolyne, Dubuque, IA, USA) set at 20 r.p.m., allowing non-adherent endometrial cells to precipitate under gravity. Fluorescence readings were taken for each well before and after washing. Each assay was run in a minimum of six replicates. The percentage of attached endometrial cells was calculated for each well [(fluorescence value after washing/fluorescence value before washing)×100].
Statistical analysis
Given normal distribution, the data are presented as mean ± standard error of the mean (SEM). Differences between treatments were assessed by t-test or one-way ANOVA with Bonferroni correction. P < 0.05 was considered statistically significant.
Results
TGF-β1 induces the expression of c-fms in endometrial cells
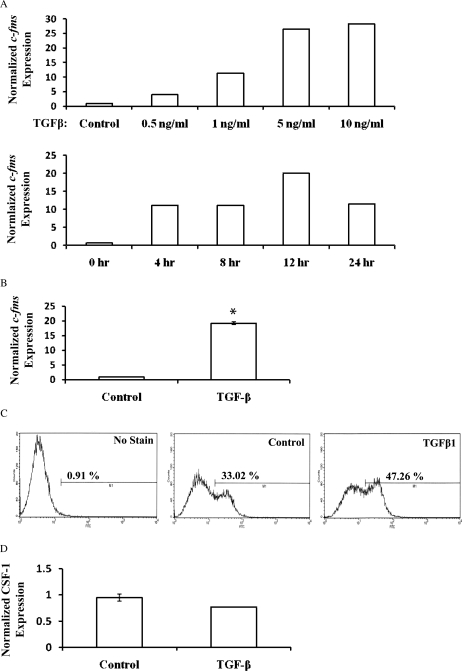
TGF-β1 induced the expression of c-fms in EM42 cells (Fig. 1). Flow cytometry confirmed EM42 cell-surface expression of c-fms, which was significantly increased after TGF-β1 treatment for 48 h (47.3% of cells) compared with control (33.0% of cells; Fig. 1C). The expression of CSF-1 was not significantly affected by TGF-β1 treatment (Fig. 1D).
Figure 1.
TGF-β1 induces c-fms expression in EM42 cells and EECs. (A) A dose–response and time course are shown for the stimulation of c-fms expression by TGF-β1. (B) EM42 cells treated with TGF-β1 at 10 ng/ml for 16 h. Real-time RT–PCR of c-fms mRNA, using the c-fms (internal) primer set, normalized to GAPDH was performed for mRNA expression analysis. Data are expressed as mean ± SEM of the gene expression adjusted to GAPDH. (C) Flow cytometry was used to detect c-fms cell-surface expression on EM42 cells. The data presented are representative of three independent experiments. (D) Real-time PCR expression of CSF-1 RNA with or without TGF-β1 treatment. (*P < 0.05 versus control.)
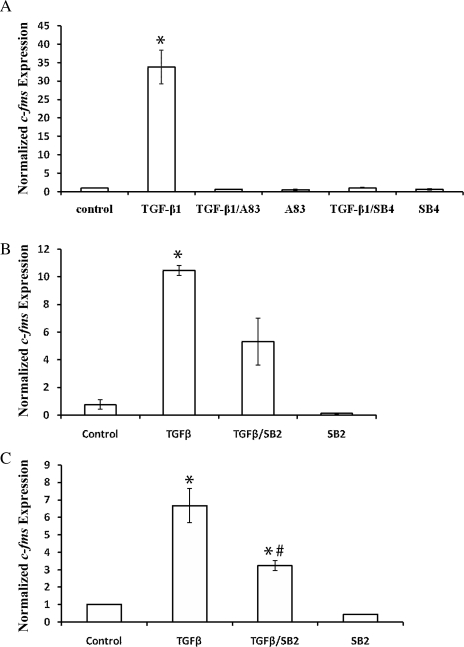
TGF-β significantly induced c-fms expression, and co-treatment with TGFBR I antagonists inhibited this induction (Fig. 2A). The p38 MAPK inhibitor SB203580 decreased c-fms induction by TGF-β1 (Fig. 2B). Similar to EM42 cells, TGF-β1 significantly induced c-fms expression in primary EECs, which was also inhibited by SB203580 (Fig. 2C).
Figure 2.
TGF-β1-induced expression of c-fms in EM42 cells and EECs is inhibited by TGFBR I antagonists and p38 inhibitor. Expression of c-fms in EM42 (A and B) and primary EECs (C) was detected by real-time RT–PCR, using the c-fms (internal) primer set that spans exons 17–20. Cells were treated with TGF-β1 (10 ng/ml) in the presence or absence of TGFBR I inhibitors A 83-01 (A83) at 5 µmol/l, SB431542 (SB4) at 10 µmol/l and SB203580 (SB2) at 10 µmol/l for 16 h. Inhibitors were added at the same time as TGF-β. Data presented are representative of three independent experiments. (*P < 0.05 versus control; #P < 0.05 versus TGF-β treatment.)
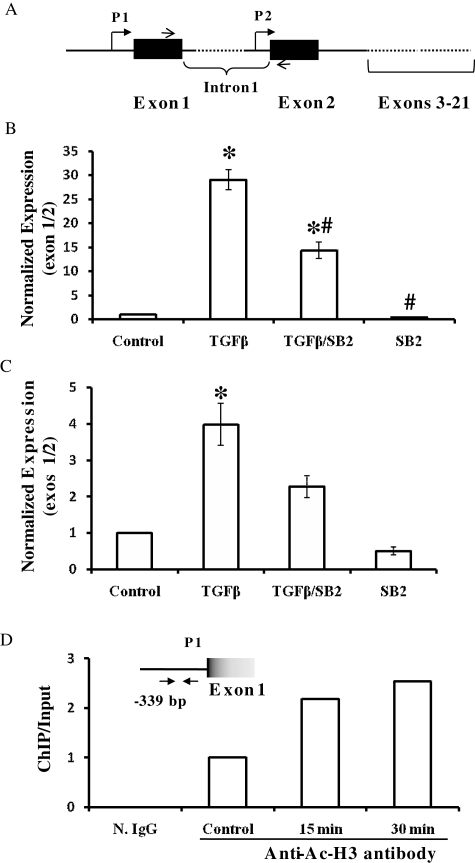
TGF-β1 regulates the expression of c-fms in endometrial cells via the induction of the trophoblast promoter of c-fms
The c-fms (internal) primer set used in RT–PCR in the previous experiments (Figs 1 and 2) detects all c-fms transcripts whether initiated by promoters 1 (trophoblast-specific) or 2 (macrophage-specific), as this primer set spans the region corresponding to exons 18 to 22. As demonstrated by real-time RT–PCR using the c-fms (exon 1/2) primer set, TGF-β1 induced the expression of c-fms mRNA containing exon 1 sequences in EM42 cells and EECs (Fig. 3). The p38 inhibitor SB203580 quenched the induction of c-fms exon 1-containing transcripts by TGF-β1 in both EM42 cells and primary EECs (Fig. 3B and C). In the ChIP assay, a 2-fold and 2.5-fold induction of c-fms promoter 1 fragments bound to Ac-H3 were observed after 15 and 30 min, respectively, following TGF-β1 treatment (Fig. 3D). Negative control immunoprecipitation with normal rabbit IgG produced no detectable signal (Fig. 3D).
Figure 3.
TGF-β1 induces c-fms expression via the trophoblast-specific promoter in EM42 and EECs. (A) A schematic representation of c-fms promoter organization: p1, promoter 1 (trophoblast specific); P2, promoter 2 (macrophage specific). Line arrows indicate the primer set (exon 1/2) corresponding to sequences in exons 1 and 2. (B and C) Real-time RT–PCR detecting the expression of c-fms exon 1. EM42 cells (B) and EECs (C) were treated with TGF-β1 at 10 ng/ml in the presence or absence of the p38 inhibitor SB203580 (10 µM) for 16 h. SB203580 was added at the same time as TGF-β. Using primer set (exon 1/2), real-time RT–PCR was used to detect c-fms mRNA containing exon 1 sequences in EM42 cells (B) and EEC (C). Data are expressed as mean ± SEM normalized to the GAPDH housekeeping gene. (*P < 0.05 versus control; #P < 0.05 versus TGF-β1 treatment.) (D) ChIP assay detecting the activity of c-fms promoter 1. EM42 cells were treated with TGF-β1 (10 ng/ml) at 15 and 30 min in addition to vehicle (control) prior to ChIP assay. ChIP assay was carried out using rabbit anti-Ac-H3 antibody (control, 15 and 30 min). N. IgG indicates normal rabbit IgG used in negative control, non-specific immunoprecipitation. Specific PCR primers (arrows) were used to detect immunoprecipitated promoter 1 (P1) region at 339 base pairs (bp) upstream of exon 1 by real-time PCR. Data are representative of three independent experiments.
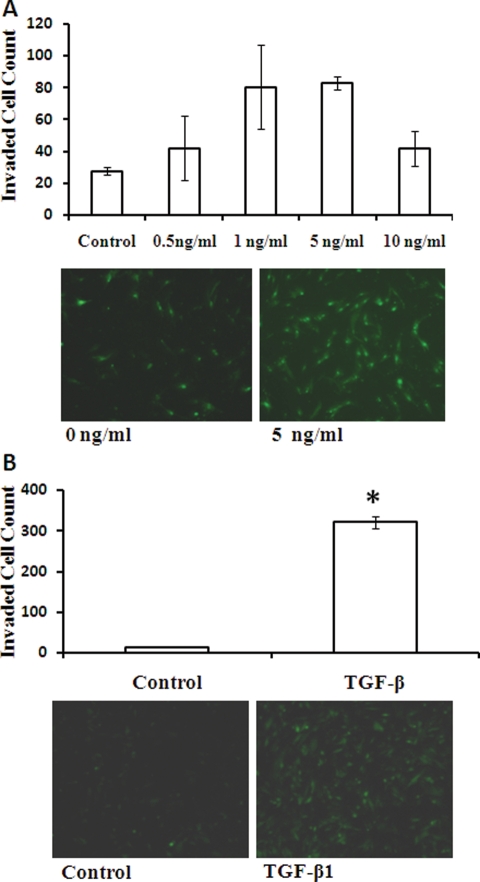
TGF-β1 induces EM42 cell and EEC invasion, which is inhibited by TGFBR I antagonist
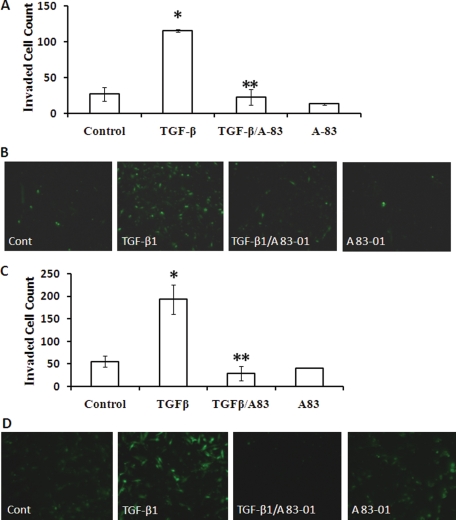
The dose–response curve for the effect of TGF-β1 on invasion is shown in Fig. 4A. TGF-β1 maximally induced EM42 invasion at 5 ng/ml by 4-fold. This dose also significantly increased the invasiveness of primary EECs (Fig. 4B). The TGF-β1-induced EM42 and EEC invasion was significantly inhibited with co-treatment with the TGFBR I antagonist A 83-01 (Fig. 5).
Figure 4.
TGF-β1 induces invasion in EM42 cells and EECs. Effects of TGF-β1 on EM42 and EEC invasion through a transmesothelial cell layer were examined. EM42 cells were treated with TGF-β1 at indicated doses prior to the invasion assay (A). Effects of TGF-β1 (5 ng/ml) on EEC invasion were also examined (B). Invasion data are expressed as mean ± SEM and representative images of invasion chambers are shown. *P < 0.05 versus control.
Figure 5.
TGFBR I inhibitor abolishes TGF-β1-induced invasion in EM42 cells and EECs. EM42 cells (A and B) and EECs (C and D) were treated with TGF-β1 (5 ng/ml) in the presence or absence of TGFBR I antagonist A 83-01 (5 µmol/l) prior to the transmesothelial invasion assay. A 83-01 was added at the same time as TGF-β. Data are presented as mean ± SEM (A and C), and representative images of invasion chambers are shown (B and D). (*P < 0.05 versus control; **P < 0.05 versus TGF-β1.)
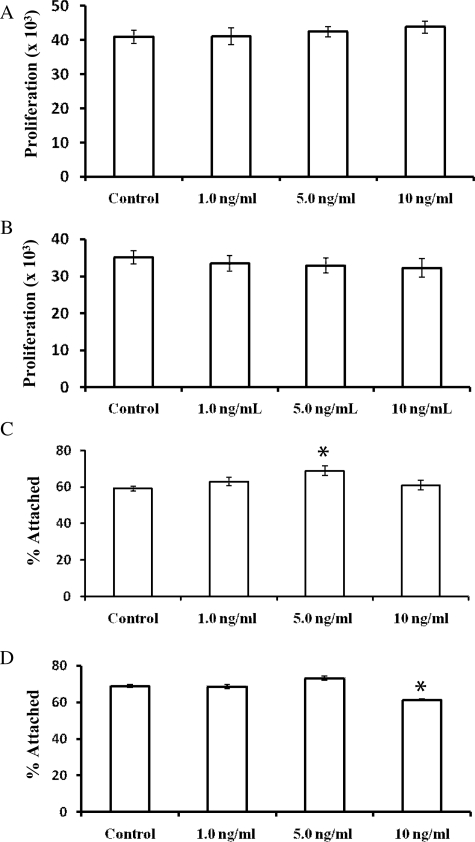
TGF-β1 does not induce cellular proliferation or attachment of EM42 cells and EECs
TGF-β1 did not affect proliferation in EM42 cells or EECs (Fig. 6A and B). A dose–response showed little effect of TGF-β1 on attachment of EM42 cells and EECs to LP9 cells (Fig. 6). A slight increase in attachment was observed in TGF-β1 (5 ng/ml)-treated EM42 cells, with 68.9% of treated cells attaching compared with 59.1% of control (Fig. 6C). TGF-β1 (5 ng/ml) increased attachment in EECs, with 73.1% of the cells attaching compared with 68.8% of control (non-significant) (Fig. 6D). A slight decrease in attachment was observed in EECs that were treated with 10 ng/ml of TGF-β1 (61.3% attached) compared with control (68.8% attached) (significant P < 0.05) (Fig. 6D).
Figure 6.
TGF-β1 has little effect on attachment of endometrial cells to PMCs and no effect on their proliferation. EM42 cells (A and C) and EECs (B and D) were treated with TGF-β1 at indicated concentrations prior to the attachment (A and B) and proliferation (C and D) assays. Data are expressed as mean ± SEM of six replicate experiments. (*P < 0.05 versus control.)
Discussion
In this study, we have examined the role of TGF-β1 in endometriosis. In addition to the effects of TGF-β1 on endometrial cell invasiveness, attachment and proliferation, we also examined whether this growth factor cross-talks with the CSF-1/c-fms pathway. Although TGF-β1 did not affect the expression of CSF-1, it did significantly increase the expression of its receptor in established and primary EECs. TGF-β1 may therefore enhance CSF-1 signaling in endometrial cells via induction of c-fms expression. Potentiating CSF-1 signaling in endometrial cells may be a mechanism by which TGF-β1 contributes to the pathogenesis of endometriosis, which may be physiologically relevant because the CSF-1/c-fms pathway has been implicated in endometriosis (Baiocchi et al., 1991). CSF-1 levels are elevated in the peritoneal fluid of endometriosis patients, and c-fms expression is increased in endometriotic implants compared with eutopic endometrium (Mettler et al., 2004). Although TGF-β1 may not be responsible for increasing CSF-1 expression, our data suggest that it may induce c-fms expression in endometriotic lesions.
Further examination of the effects of TGF-β1 on c-fms expression suggested that the c-fms trophoblast promoter may be activated by TGF-β1. This is suggested by RT–PCR experiments, using a primer set that detects exon 1 expression, and the ChIP assay detecting increased Ac-H3 levels at the c-fms promoter 1 region. The role of p38 MAPK in mediating TGF-β1 induction of c-fms expression was suggested by the use of the inhibitor SB203580. Further studies are required to delineate the mechanistic actions of TGF-β1 signaling and p38 on the c-fms trophoblast promoter. Such studies may identify therapeutic targets within the TGF-β1 pathway that may blunt CSF-1 signaling by reducing c-fms expression in endometriotic lesions.
Our data demonstrated that TGF-β1 significantly increased EEC invasion, which suggests a role for elevated TGF-β1 levels in the peritoneal fluid of endometriosis patients (Oosterlynck et al., 1994). Although the CSF-1/c-fms pathway may mediate this action by TGF-β in endometrial cells, other factors/pathways induced by TGF-β may also be involved in enhanced invasiveness. These include TGF-β1-regulated metalloproteases that degrade the extracellular matrix and allow for cellular motility and invasion (Van Themsche et al., 2007). We also showed that TGF-β1 did not affect cellular proliferation of EM42 cells and EECs, suggesting that TGF-β1 may not have an effect on cellular growth in endometriosis. This is consistent with published data showing that TGF-β3 induced the invasiveness of endometrial cancer cells, but not proliferation (Van Themsche et al., 2007). TGF-β1 had little effect on EEC attachment to mesothelial cells. High level of EEC attachment to PMCs was observed without TGF-β1 treatment, suggesting that other factors are involved in mediating cellular attachment. For example, published evidence has suggested that the cell-surface receptor CD44 may be involved in endometrial cell attachment to PMCs (Griffith et al., 2009).
Our data show that antagonists of TGFBR I are potent inhibitors of TGF-β1-induced transmesothelial invasion, suggesting that such inhibitors may be useful in the treatment of endometriosis. The inhibitor A 83-01 not only antagonizes TGFBR I but also other receptor members of the TGF-β super-family: alk4 (activin A receptor type 1B) and alk 7 (activin A receptor type 1C). It is possible that the A 83-01 may act on the activin pathway as well. We previously showed that activin enhanced EEC invasion (Ferreira et al., 2008), although to a lesser extent than TGF-β1 in this study. TGF-β inhibitors are being tested in clinical trials with metastatic melanoma and renal cell carcinoma (Wrzesinski et al., 2007). LY364947, a TGFBR I inhibitor, was shown to be effective in inhibiting xenograft pancreatic adenocarcinoma cell tumor development. The TGFBR I inhibitor SD208 inhibited the growth and invasiveness of pancreatic cancer cells in vitro and in vivo (Gaspar et al., 2007; Medicherla et al., 2007). Further studies are required to elucidate the mechanistic actions of TGF-β and its antagonists on the invasiveness of endometrial cells. Caution needs to be taken, however, when designing drugs that target TGF-β signaling because of undesirable side effects that may arise due to potentially blocking tumor-suppressor activity of TGF-β (Jakowlew, 2006; Tian and Schiemann, 2009). By dissecting TGF-β signaling, our studies and others like it may lead to the development of improved therapeutics that target TGF-β-signaling components involved in endometriosis without affecting its tumor-suppressor activity.
In conclusion, our findings that TGF-β1 induces c-fms expression suggest that TGF-β1 enhances CSF-1 signaling by increasing the expression of its receptor. We present novel evidence suggesting that TGF-β1, which has been shown to be elevated in the peritoneal fluid of endometriosis patients and activated in endometriotic cysts, may enhance the invasive characteristics of EECs. Our data raise the possibility that inhibitors of TGF-β-signaling components, whether receptors or downstream signaling molecules, may be efficacious in the treatment of endometriosis.
Funding
This work was supported by the National Institutes of Health (CA P30CA54174 and R01 HD049637-01A2 to R.R.T.) and the University Research Council, University of Texas Health Science Center at San Antonio (10003554 to N.B.K.).
Acknowledgements
The authors would like to acknowledge the technical assistance by Sumalatha Mummadisetti. Flow cytometry was performed in the flow cytometry core at Cancer Therapy and Research Center, the University of Texas Health Science Center at San Antonio, San Antonio, TX, USA.
References
- Baiocchi G, Kavanagh JJ, Talpaz M, Wharton JT, Gutterman JU, Kurzrock R. Expression of the macrophage colony-stimulating factor and its receptor in gynecologic malignancies. Cancer. 1991;67:990–996. doi: 10.1002/1097-0142(19910215)67:4<990::aid-cncr2820670422>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Dennler S, Goumans MJ, ten Dijke P. Transforming growth factor beta signal transduction. J Leukoc Biol. 2002;71:731–740. [PubMed] [Google Scholar]
- Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24:235–258. doi: 10.1016/s0889-8545(05)70302-8. [DOI] [PubMed] [Google Scholar]
- Ferreira MC, Witz CA, Hammes LS, Kirma N, Petraglia F, Schenken RS, Reis FM. Activin A increases invasiveness of endometrial cells in an in vitro model of human peritoneum. Mol Hum Reprod. 2008;14:301–307. doi: 10.1093/molehr/gan016. [DOI] [PubMed] [Google Scholar]
- Gaspar NJ, Li L, Kapoun AM, Medicherla S, Reddy M, Li G, O'Young G, Quon D, Henson M, Damm DL, et al. Inhibition of transforming growth factor beta signaling reduces pancreatic adenocarcinoma growth and invasiveness. Mol Pharmacol. 2007;72:152–161. doi: 10.1124/mol.106.029025. [DOI] [PubMed] [Google Scholar]
- Gill K, Kirma N, Gunna VS, Santanam N, Parthasarathy S, Tekmal RR. Regulation of colony stimulating factor-1 (CSF-1) in endometrial cells: glucocorticoids and oxidative stress regulate the expression of CSF-1 and its receptor c-fms in endometrial cells. Fertil Steril. 2001;76:1005–1011. doi: 10.1016/s0015-0282(01)02735-2. [DOI] [PubMed] [Google Scholar]
- Griffith JS, Liu YG, Tekmal RR, Binkley PA, Holden AE, Schenken RS. Menstrual endometrial cells from women with endometriosis demonstrate increased adherence to peritoneal cells and increased expression of CD44 splice variants. Fertil Steril. doi: 10.1016/j.fertnstert.2008.12.012. (February 5, 2009) 10.1016/j.fertnstert.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- Hume DA, Yue X, Ross IL, Favot P, Lichanska A, Ostrowski MC. Regulation of CSF-1 receptor expression. Mol Reprod Dev. 1997;46:46–52. doi: 10.1002/(SICI)1098-2795(199701)46:1<46::AID-MRD8>3.0.CO;2-R. [Discussion 52–53] [DOI] [PubMed] [Google Scholar]
- Jakowlew SB. Transforming growth factor-beta in cancer and metastasis. Cancer Metastasis Rev. 2006;25:435–457. doi: 10.1007/s10555-006-9006-2. [DOI] [PubMed] [Google Scholar]
- Jensen JR, Witz CA, Schenken RS, Tekmal RR. A potential role for colony-stimulating factor 1 in the genesis of the early endometriotic lesion. Fertil Steril. doi: 10.1016/j.fertnstert.2008.09.050. (November 4, 2008) 10.1016/j.fertnstert.2008.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RL, Stoikos C, Findlay JK, Salamonsen LA. TGF-beta superfamily expression and actions in the endometrium and placenta. Reproduction. 2006;132:217–232. doi: 10.1530/rep.1.01076. [DOI] [PubMed] [Google Scholar]
- Kirma N, Hammes LS, Liu YG, Nair HB, Valente PT, Kumar S, Flowers LC, Tekmal RR. Elevated expression of the oncogene c-fms and its ligand, the macrophage colony-stimulating factor-1, in cervical cancer and the role of transforming growth factor-beta1 in inducing c-fms expression. Cancer Res. 2007;67:1918–1926. doi: 10.1158/0008-5472.CAN-06-1991. [DOI] [PubMed] [Google Scholar]
- Komiyama S, Aoki D, Komiyama M, Nozawa S. Local activation of TGF-beta1 at endometriosis sites. J Reprod Med. 2007;52:306–312. [PubMed] [Google Scholar]
- Loverro G, Maiorano E, Napoli A, Selvaggi L, Marra E, Perlino E. Transforming growth factor-beta 1 and insulin-like growth factor-1 expression in ovarian endometriotic cysts: a preliminary study. Int J Mol Med. 2001;7:423–429. doi: 10.3892/ijmm.7.4.423. [DOI] [PubMed] [Google Scholar]
- Luo X, Xu J, Chegini N. The expression of Smads in human endometrium and regulation and induction in endometrial epithelial and stromal cells by transforming growth factor-beta. J Clin Endocrinol Metab. 2003;a 88:4967–4976. doi: 10.1210/jc.2003-030276. [DOI] [PubMed] [Google Scholar]
- Luo X, Xu J, Chegini N. Gonadotropin releasing hormone analogue (GnRHa) alters the expression and activation of Smad in human endometrial epithelial and stromal cells. Reprod Biol Endocrinol. 2003;b 1:125. doi: 10.1186/1477-7827-1-125. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Medicherla S, Li L, Ma JY, Kapoun AM, Gaspar NJ, Liu YW, Mangadu R, O'Young G, Protter AA, Schreiner GF, et al. Antitumor activity of TGF-beta inhibitor is dependent on the microenvironment. Anticancer Res. 2007;27:4149–4157. [PubMed] [Google Scholar]
- Mettler L, Schmutzler AG, Koch K, Schollmeyer T, Salmassi A. Identification of the M-CSF receptor in endometriosis by immunohistochemistry and RT-PCR. Am J Reprod Immunol. 2004;52:298–305. doi: 10.1111/j.1600-0897.2004.00228.x. [DOI] [PubMed] [Google Scholar]
- Nair AS, Nair HB, Lucidi RS, Kirchner AJ, Schenken RS, Tekmal RR, Witz CA. Modeling the early endometriotic lesion: mesothelium-endometrial cell co-culture increases endometrial invasion and alters mesothelial and endometrial gene transcription. Fertil Steril. 2008;90:1487–1495. doi: 10.1016/j.fertnstert.2007.09.047. [DOI] [PubMed] [Google Scholar]
- Oosterlynck DJ, Meuleman C, Waer M, Koninckx PR. Transforming growth factor-beta activity is increased in peritoneal fluid from women with endometriosis. Obstet Gynecol. 1994;83:287–292. [PubMed] [Google Scholar]
- Roberts WM, Shapiro LH, Ashmun RA, Look AT. Transcription of the human colony-stimulating factor-1 receptor gene is regulated by separate tissue-specific promoters. Blood. 1992;79:586–593. [PubMed] [Google Scholar]
- Sapi E, Flick MB, Gilmore-Hebert M, Rodov S, Kacinski BM. Transcriptional regulation of the c-fms (CSF-1R) proto-oncogene in human breast carcinoma cells by glucocorticoids. Oncogene. 1995;10:529–542. [PubMed] [Google Scholar]
- Tian M, Schiemann WP. The TGF-beta paradox in human cancer: an update. Future Oncol. 2009;5:259–271. doi: 10.2217/14796694.5.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Themsche C, Mathieu I, Parent S, Asselin E. Transforming growth factor-beta3 increases the invasiveness of endometrial carcinoma cells through phosphatidylinositol 3-kinase-dependent up-regulation of X-linked inhibitor of apoptosis and protein kinase c-dependent induction of matrix metalloproteinase-9. J Biol Chem. 2007;282:4794–4802. doi: 10.1074/jbc.M608497200. [DOI] [PubMed] [Google Scholar]
- Vassiliadis S, Relakis K, Papageorgiou A, Athanassakis I. Endometriosis and infertility: a multi-cytokine imbalance versus ovulation, fertilization and early embryo development. Clin Dev Immunol. 2005;12:125–129. doi: 10.1080/17402520500125484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanichkul T, Han S, Huang RP, Sidell N. Cytokine regulation by peroxisome proliferator-activated receptor gamma in human endometrial cells. Fertil Steril. 2003;79(Suppl. 1):763–769. doi: 10.1016/s0015-0282(02)04835-5. [DOI] [PubMed] [Google Scholar]
- Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clin Cancer Res. 2007;13:5262–5270. doi: 10.1158/1078-0432.CCR-07-1157. [DOI] [PubMed] [Google Scholar]