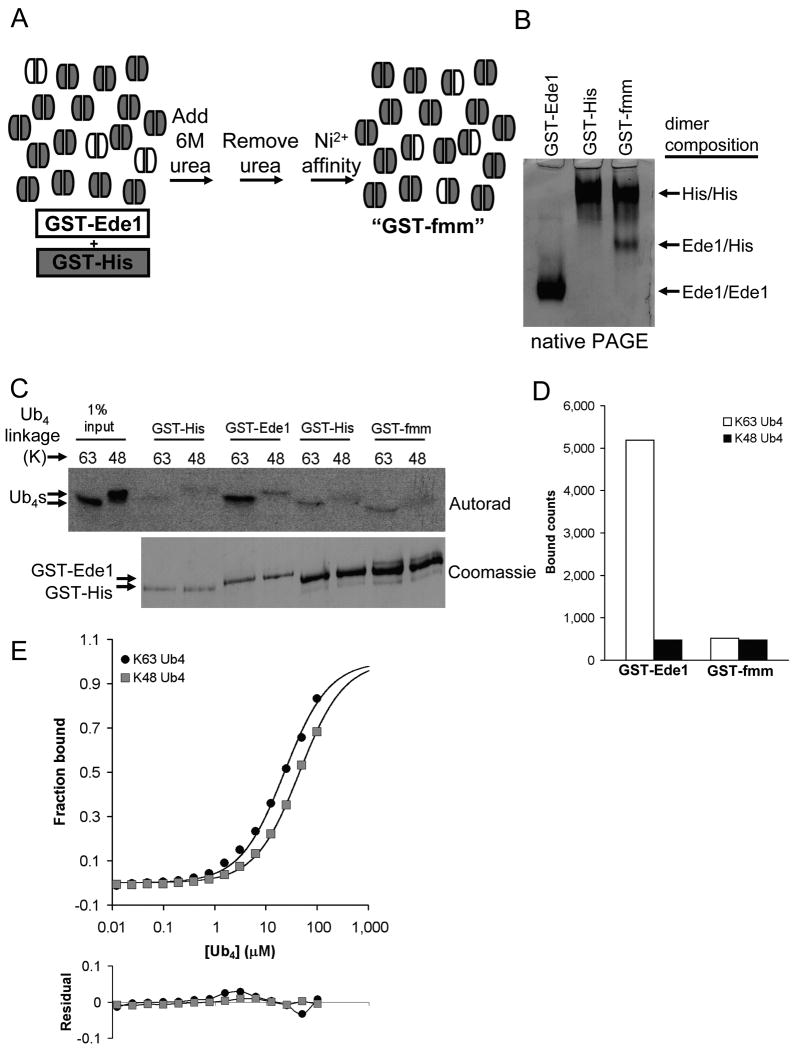
Figure 4.
The bivalency of GST-Ede1 UBA preferentially promotes binding to K63-polyUb over K48-polyUb. (a) The process of making the functionally monomeric version of GST-Ede1 (GST-fmm) from a mixture of GST-His (gray subunits) and GST-Ede1 (white subunits) is shown schematically. (b) GST subunit exchange can be seen by comparing the starting materials to the final GST-fmm mixture after separation by native PAGE. The heterodimer has an intermediate pI and migrates between the positions of the two homodimers. (c) GST-Ede1 and GST-fmm were used to pull-down 125I-labeled Ub4 chains. The upper panel shows an autoradiogram of SDS-PAGE-separated Ub4 chains. To resolve GST-Ede1 and GST-His by SDS-PAGE, smaller amounts of the same samples were separated for a longer time on a second gel (lower panel). The amounts of GST-Ede1 and GST-fmm used in the assay were adjusted to contain similar amounts of the GST-Ede1 subunit (upper band). Separate negative controls (GST-His) were performed to account for different amounts of total protein on the GST-Ede1 and GST-fmm beads. The results of two assays are averaged in (d). (e) SPR analysis confirms this result. K63-Ub4 affinity and selectivity were reduced with GST-fmm (upper panel) relative to the GST-Ede1 homodimer. Data shown are an average from two independent trials that differed by <10%. Deviations from the fits are shown in the lower panel.