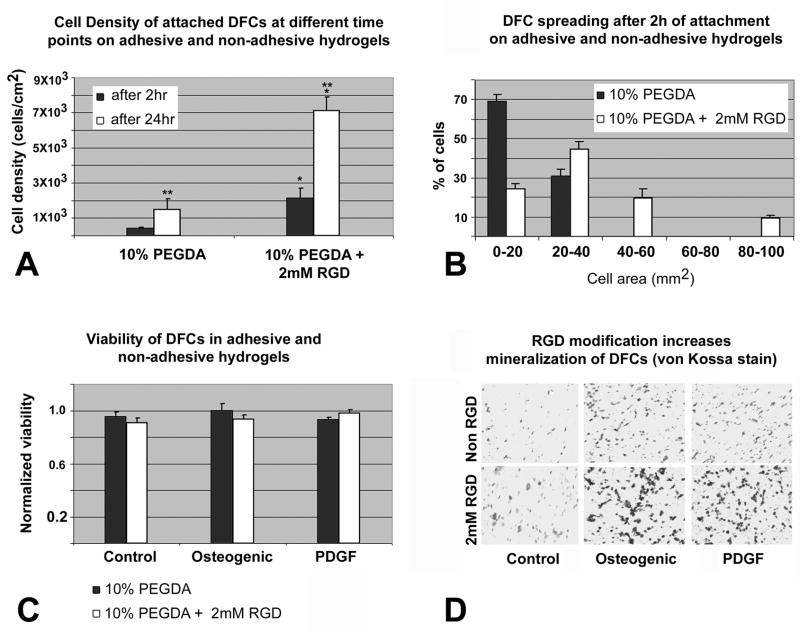
Fig 5. Attachment, spreading, viability and mineralization of DFCs depending on RGD-modification of PEGDA hydrogels.
Fig. 5A. Cell density of attached DFCs after 2 and 24 hrs on 10% PEGDA without Acr-PEG-RGD or containing 2mM Acr-PEG-RGD. Values are reported as an average of three random fields times three hydrogel wells at each time point. There was a statistical difference (p<0.05) in the number of DFCs attached on the two types of hydrogels at the same time point (**) and on the same type of hydrogel at different time points (*). Fig. 5B. Histogram of DFC spreading after 2 hr attachment on either 10% PEGDA or 10% PEGDA with 2mM Acr-PEG-RGD. To visualize the morphology of the attached cells on these two types of hydrogel surfaces, micrographs were obtained following 2 hrs of attachment, and the area of cell spreading was calculated by measuring the area individual cell occupied using the NIH imaging software. Values are reported as the percentage of the attached cells spreading and occupying areas in the range specified (0–100 mm2) over three random fields per composition. Fig. 5C. Viability of DFCs in 10% PEGDA with or without 2mM of Acr-PEG-RGD after 3 days culture in osteogenic differentiation medium, PDGF supplemented medium, and control medium. Values are reported as the average of three constructs per composition per treatment. There was no statistical difference (p>0.05) in the viability of DFCs between the modified and unmodified hydrogels and their treatment groups. Fig. 5D. Von Kossa stained sections of untreated (control) and treated (osteogenic, PDGF) DFCs encapsulated in unmodified (upper row) and RGD-modified (lower row) hydrogels after 2 weeks of in vitro culture. Mineralization was significantly increased in the RGD-modified group as seen by dense mineralization nodules.