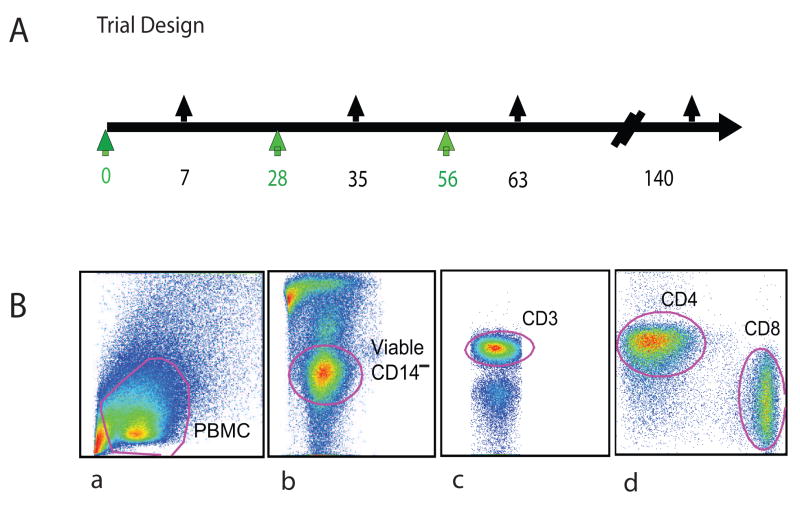
Figure 1.
(A) Study Design. AMA1-C1 (FVO+3D7) formulated with Alhydrogel® were administered at a dose of 80 μg IM to healthy American volunteers. Black arrows on the top of the time line indicate blood sampling time points in vaccinees. (B) The gating strategy for the analysis of cytometry data. For PBMC, cell debris was excluded first (a), followed by exclusion of non-viable cells and monocytes (b); the lymphocytes were identified (c), and CD4 and CD8 markers were then used to identify two populations for further characterization (d).