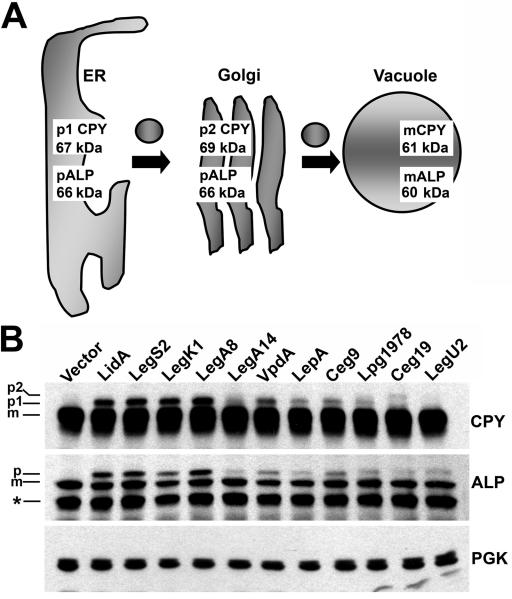
Fig. 3. Confirmed and candidate Dot/Icm substrates cause defects in the trafficking of secretory proteins to the yeast vacuole.
(A) Schematic of CPY and ALP trafficking from the ER to the vacuole in yeast. CPY and ALP undergo organelle specific post-translational modifications during trafficking from the ER to the Golgi and finally the vacuole via vesicular intermediates. These modifications alter the molecular weights of CPY and ALP and enable monitoring of their trafficking by immunoblot. (B) Analysis of CPY and ALP trafficking in yeast strains producing L. pneumophila proteins. Yeast strains were grown in media containing 2% galactose to induce production of L. pneumophila proteins (Experimental Procedures). Samples were analyzed by immunoblot with antibodies directed against CPY and ALP. PGK immunoblot is a control to show equivalent loading. The positions of the mature and precursor forms of CPY and ALP are indicated. Asterisk indicates an ALP degradation product. A LidA producing strain is included as a positive control for a protein known to cause secretory defects (Derre and Isberg, 2005).