Table 1.
Catalyst screening and reaction condition optimization for the two-component reactiona
 | |||||
|---|---|---|---|---|---|
| Entry | Catalyst | Solvent | Time (h) | Yieldb (%) | eec (%) |
| 1 | 1 | CH2Cl2 | 27 | 80 | 23 |
| 2 | 2 | CH2Cl2 | 24 | 92 | 96 |
| 3 | 3 | CH2Cl2 | 24 | 91 | 65 |
| 4 | 4 | CH2Cl2 | 27 | 80 | 0 |
| 5 | 5 | CH2Cl2 | 27 | 88 | 14 |
| 6 | 6 | CH2Cl2 | 24 | 79 | 11 |
| 7 | 7 | CH2Cl2 | 27 | 96 | 10 |
| 8 | 8 | CH2Cl2 | 27 | 92 | 22d |
| 9 | 9 | CH2Cl2 | 24 | 83 | 6d |
| 10 | 2 | CHCl3 | 20 | 87 | 92 |
| 11 | 2 | THF | 20 | 74 | 60 |
| 12 | 2 | Et2O | 20 | 94 | 62 |
| 13 | 2 | Benzene | 20 | 79 | 82 |
| 14 | 2 | CH3CN | 20 | 91 | 24 |
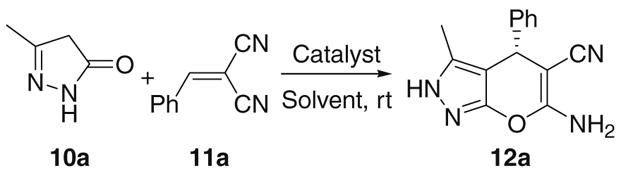
All reactions were carried out with 10a (0.10 mmol), 11a (0.10 mmol), and the catalyst (5 mol %) in the indicated solvent (2.0 mL) at rt.
Yield of isolated product after chromatography.
Determined by HPLC analysis on a ChiralPak AS column.
The S enantiomer was obtained as the major product.
