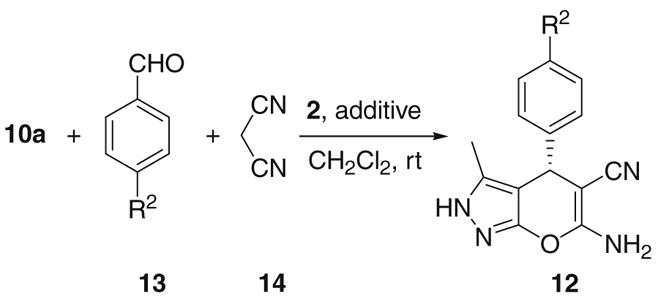
Table 3.
Enantioselective three-component reaction for the synthesis of pyranopyrazoles with catalyst 2a
 | |||||
|---|---|---|---|---|---|
| Entry | 12 | Additive | Time (h) | Yieldb (%) | eec (%) |
| 1 | 12a | — | 18 | 80 | 96 |
| 2 | 12a | Na2SO4d | 25 | 69 | 99 |
| 3 | 12a | MS(4 Å)e | 21 | 72 | 94 |
| 4 | 12c | — | 6 | 54 | 42 |
| 5 | 12c | Na2SO4d | 6 | 50 | 34 |
| 6 | 12c | MS(4 Å)e | 6 | 50 | 70 |
| 7 | 12d | — | 6.5 | 74 | 66 |
| 8 | 12d | Na2SO4d | 23 | 88 | 25 |
| 9 | 12d | MS(4 Å)e | 22 | 89 | 58 |
All reactions were carried out with 10a (0.10 mmol), 13 (0.10 mmol), 14 (0.10 mmol), and the catalyst (5 mol %) in CH2Cl2 (2.0 mL) at rt.
Yield of isolated product after chromatography.
Determined by HPLC analysis on a ChiralPak AS column.
Na2SO4 (0.10 mmol) was added.
Molecular sieves (40 mg) were added.
