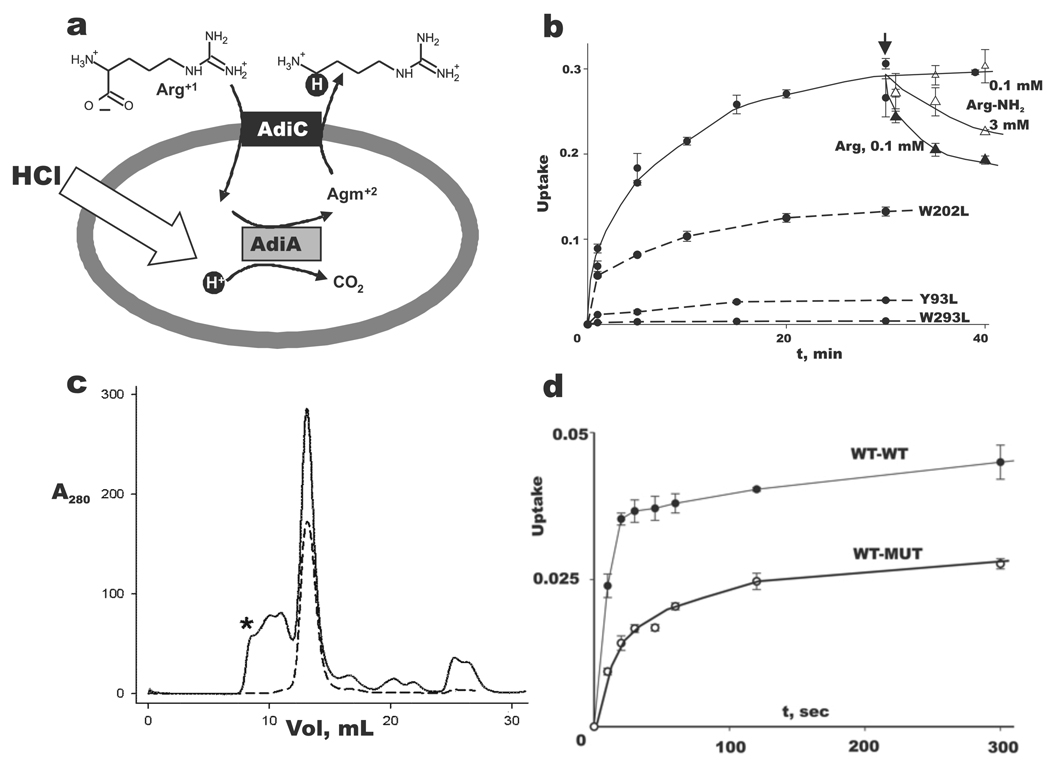
Fig 1. AdiC physiology and function.
a. Virtual proton pumping in extreme acid resistance. Schematic of Arg-dependent acid resistance in E. coli, with AdiC-mediated Arg-Agm exchange across the inner membrane coupled to acid-activated Arg-decarboxylase AdiA. Virtual proton is shown in black circle.
b. Selection of α-carboxylate for transport. Uptake (fraction of total counts added) of 14C-Arg at 50 µM external concentration into AdiC-reconstituted liposomes (2 µg/mg lipid) was followed for 30 min, and then either Arg (filled triangles) or Arg-NH2 (open triangles) was added (arrow) externally to the indicated concentration. Additional Arg uptake experiments used the indicated AdiC mutants (dashed curves). Error bars indicate s.e.m. of triplicate experiments.
c. Proper assembly of tandem construct. Size-exclusion profiles of purified homodimeric AdiC (dashed trace) and WT-WT tandem (solid trace) in its final purification step immediately after elution from Co-affinity column. Material eluting between void volume of Superdex-200 column (asterisk) and main peak most likely represents improperly assembled, oligomeric tandems. Identical profile is obtained with WT-MUT tandem in which the second subunit contains the W293L mutation (data not shown). d. A half-dead heterodimer is functionally active. Arg uptake timecourses for WT-WT to WT-MUT tandems reconstituted at 0.2 µg/mg lipid, a low protein density where transporting liposomes carry only one copy to the reconstituted protein6,32. WT-WT transport rate is ~70% of normal homodimeric AdiC (data not shown).