Figure 1. HSP90 inhibitor 17-DMAG promotes the loss of tumor EphA2 protein (via degradation) in a dose-, time- and proteasome-dependent manner.
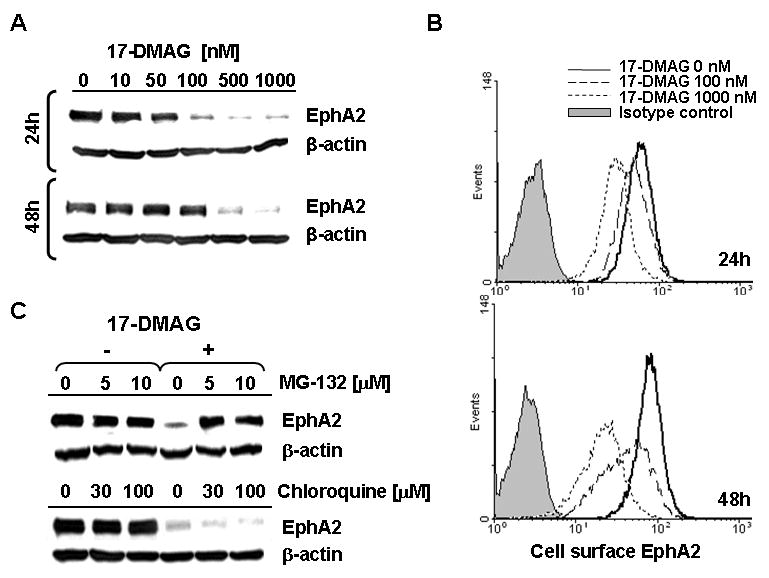
A. The EphA2+ SLR20 RCC line was incubated in the absence or presence of 17-DMAG (10-1000 nM) for 24h or 48h at 37°C, before generation of cell lysates and Western Blot analysis to determine levels of EphA2 protein expression. β-actin was monitored as an internal control protein. B. SLR20 cells were treated as above, with cell surface expression of EphA2 protein monitored by flow cytometry. Differences in tumor cell MFI expression of EphA2 were significant for 17-DMAG-treated vs. control, untreated tumor cells evaluated in flow-based assays (i.e. p = 0.008 at 24h for 500 nM 17-DMAG treated (MFI = 28 +/- 13) vs. untreated (MFI = 60 +/- 12). C. SLR20 cells were treated with 500 nM 17-DMAG in the absence or presence of MG-132 (10 μM) or chloroquine (100 μM) prior to Western Blot analysis in order to analyze the dependency of EphA2 (vs. control β-actin) protein loss on proteasome function or endosomal acidification, respectively. Data in each panel are representative of 4 independent experiments performed.
