Fig. 1.
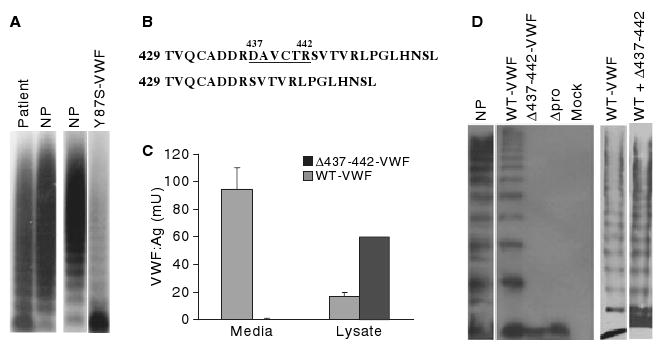
Plasma von Willebrand factor (VWF) multimer analysis, mutation identification, and expression of variant VWF. (A) Patient plasma (lane 1), normal human plasma (NP) and plasma from a previously reported VWD patient were analyzed by sodium dodecylsulfate (SDS)--agarose gel electrophoresis and western blotting [6]. (B) A mutation identified in the D2 domain of VWF propeptide (VWFpp) predicts deletion of amino acids 437--442. (C) VWF concentration in conditioned media and cell lysates from HEK293T cells expressing wild-type (WT) VWF (gray bars) and Δ437--442-VWF (black bars) from three separate transfections with each vector expressed in duplicate. Results shown are mean VWF levels, with error bars indicating standard deviation. (D) Multimeric composition of recombinant VWF proteins (2% SDS--agarose): normal human plasma (lane 1), wild-type VWF (lanes 2 and 6), Δ437--442-VWF (lane 3), propeptide-deleted VWF (Δpro, lane 4), mock-transfected cells (lane 5), coexpressed wild-type VWF and Δ437--442-VWF (lane 7). The Δ437--442-VWF mutation results in decreased VWF secretion and defective multimerization.
