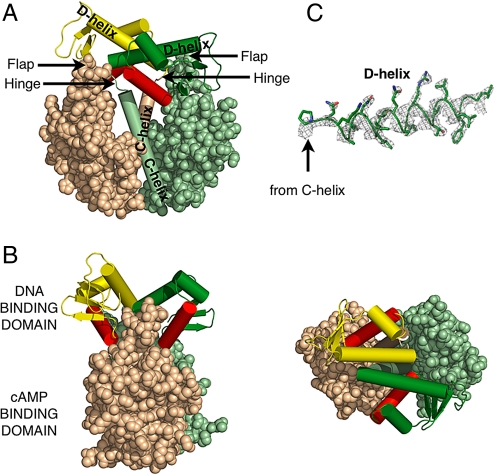
Fig. 1.
Structure of the unliganded D138L CAP mutant. The DNA binding domain of one monomer is colored forest, whereas the other is yellow. The DNA recognition helices are colored red. The cAMP binding domain of one monomer is colored pale green, whereas the other is colored wheat. This color scheme is used in all other figures unless noted. (A) The nucleotide binding domains are represented as spheres, whereas the C-helices and the helices of the DNA binding domains are represented as cylinders. (B) Alternative views of the mutant are shown. Rotation of the molecule by 90° along its central vertical and horizontal axis, respectively, produces the “side view” (Left) and “top view” (Right). (C) Electron density corresponding to the D-helix, contoured at 2σ, that was calculated using experimental phases and observed amplitudes to 2.4-Å resolution.