Figure 2.
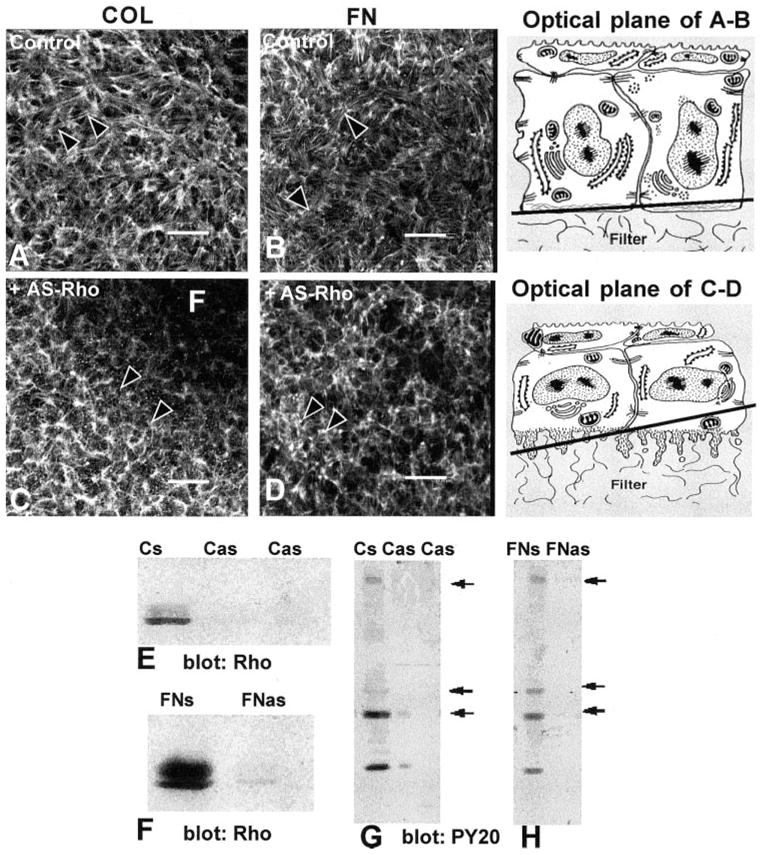
Corneal epithelia transiently transfected (24 hours) with antisense Rho oligonucleotides (10-mer, Fig. 1L-C) were stimulated with FN or COL for 2 hours and stained with fluorescent phalloidin. All experiments and procedures were repeated at least three times with similar results. Single confocal optical sections through basal cells, as illustrated in the tissue schematic, show the distribution of the F-actin in epithelia stimulated with either COL or FN in the control tissues (A, B). Areas that did not contain actin staining (dark) were optical sections through the supporting filter (F). The tissues were not flat; therefore, a single optical section may contain the filter (F) and basal cell cytoplasm (C). In tissues transiently transfected with Rho antisense (C, D), the epithelia had decreased actin in the basal compartment and did not form an ACM after stimulation with COL or FN. The optical sections were taken at the base of the epithelia, as illustrated in the schematic at various tangential planes. Epithelia harvested and analyzed with Western blot analysis were probed with anti-Rho (E, F). The sense control (FNs or Cs) had a 21-kDa protein that migrated as a doublet. In contrast, epithelia transfected with antisense Rho had 80% less Rho protein (E, F; Cas, FNas). To determine whether Rho protein affects other signal transduction proteins, the same Western blot analysis used in (E) and (F) were stripped and reprobed with anti-phosphotyrosine (G, H). In control tissues (Cs, FNs), tyrosine-phosphorylated proteins at molecular weights of approximately 190, 68, 42, and 20 kDa were detectable (arrows), whereas in the tissue treated with antisense Rho oligonucleotides (Cas, FNas), these proteins were significantly decreased. Scale bar, 20 μm.
