Figure 5.
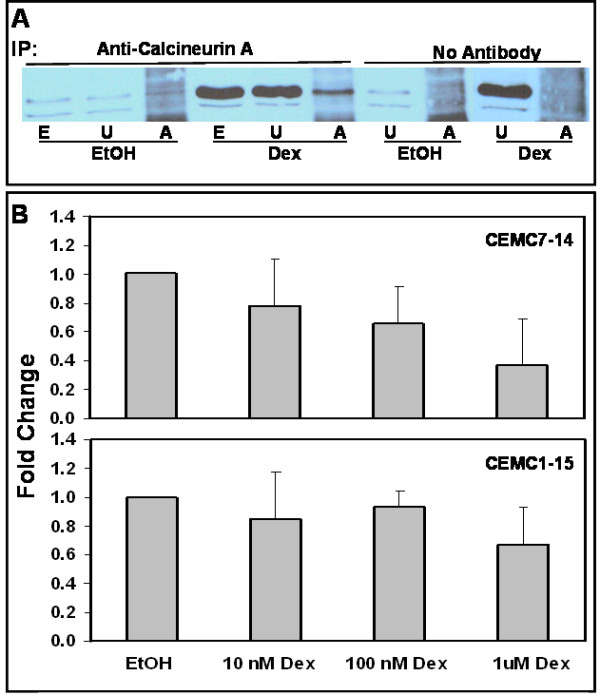
Calcineurin binds to RCAN1-1 in correlation with loss of phosphatase activity: Panel A: CEM-C7-14 cells were treated with ethanol vehicle (EtOH) or 100 nM Dex (Dex) for 24 h, cell lysates were prepared in RIPA buffer and subjected (500 μg protein) to immunoprecipitation with either anti-calcineurin A antibody or no antibody, as indicated. Unadsorbed lysates (U; 50 μg protein) or proteins bound to Protein A-Agarose (A; entire pellet) were run alongside total cell lysates (E; 50 μg protein). Western blotting was performed using RCAN1 antibody (Abgent # AP6315c). Panel B: CEM-C7-14 or CEM-C1-15 cells treated with 0-1 μM Dex were subjected to a calcineurin phosphatase activity assay using a kit (Biomol). Cells were lysed in the lysis buffer provided, and were cleared of any free phosphate by passing through Micro Bio-Spin P-30 Tris chromatography columns (Bio-Rad). Phosphate free lysates were incubated with a known calcineurin substrate phosphopeptide, RII, and the free phosphate released was measured using a Malachite Green based assay. Okadaic acid was used to inhibit PP1 and PP2A and Okadaic acid and EGTA was used to block PP1, PP2A and PP3C (calcineurin) activity. To calculate PP3C activity, okadaic acid inhibited activity was subtracted from total phosphatase activity. Alternatively, okadaic acid and EGTA inhibited activity was subtracted from okadaic acid inhibited activity. Phosphate released in nmoles was corrected for protein content, measured using the Bradford assay. Data are averages ± SD of two (CEM-C1-15) or three (CEM-C7-14) independent treatments, each normalized to the ethanol control.
