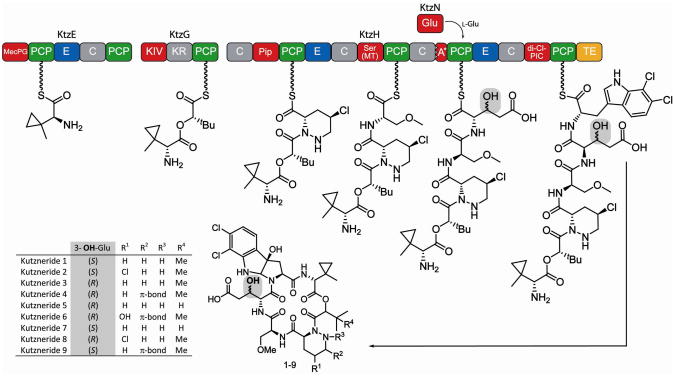
Figure 1.
Proposed biosynthesis and structures of kutznerides 1–9. Adenylation (A) domains are shown in red, peptidyl carrier protein (PCP) domains in green, condensation (C) domains in grey and epimerization (E) domains in blue. The third A domain of KtzH (A*) is only 88-aa in size and nonfunctional. The thioesterase (yellow) is proposed to cleave the mature kutzneride from the assembly line by cyclization and thereby yield kutznerides 1–9. The site of glutamic acid hydroxylation is highlighted in grey. All known kutzneride species where found to contain D-β-hydroxylated glutamic acid either in the erythro (1, 2, 7, 9) or threo (3–6, 8) form.