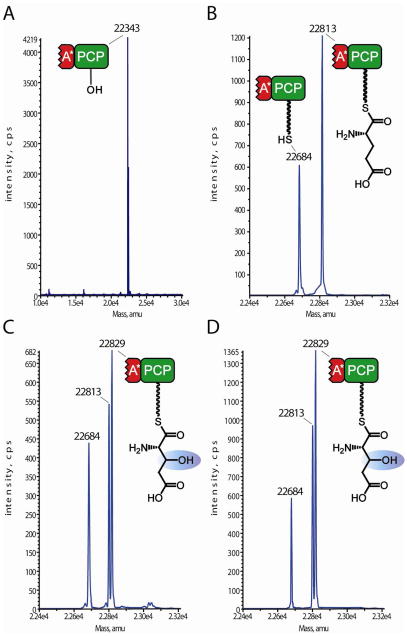
Figure 2.
QTOF-MS analysis of hydroxylation activity of KtzO and KtzP on PCP-tethered glutamic acid. A: apo-A*PCP3 with an apparent mass of 22343 Da. B, control reaction: A*PCP3 loaded with synthetic coenzyme A glutamic acid by Sfp and incubated for 2h. The mass shift of 470 Da shows that ppant-S-Glu was transferred on the PCP domain. C: A*PCP3 after incubation with αKG, (NH4)2Fe(SO4)2 and KtzO, preceded by incubation with Sfp and CoA-S-Glu. The mass difference of 16 Da compared with B indicates hydroxylation of the PCP-bound glutamic acid. D: Same as C, but after incubation with KtzP. Hydrolysis is observed for B and C and D, and the mass of 22684 Da corresponds to the holo-A*PCP3.