Abstract
Purpose
To describe the practical technical aspects of magnetic resonance imaging (MRI) and magnetic resonance spectroscopic imaging (MRSI) and to summarize the current and potential future status of MRI and MRSI in the localization, staging, treatment planning, and post-treatment follow-up of prostate cancer.
Technique
Published contemporary series of patients with prostate cancer evaluated by MRI and MRSI before or after radiation therapy were reviewed, with particular respect to the role of MRI and MRSI in treatment planning, outcome prediction, and detecting local recurrence.
Results
Volumetric localization is of limited accuracy for tumors less than 0.5 cm3. Staging by MRI, which is improved by the addition of MRSI, is of incremental prognostic significance in patients with moderate and high-risk tumors. The finding of more than 5 mm of extracapsular extension prior to radiation seems to be of particular negative prognostic significance, and the latter group may be candidates for more aggressive supplemental therapy. The use of MRI to assist radiation treatment planning has been shown to improve outcome. MRSI may be helpful in the detection of local recurrence after radiation.
Conclusions
Only MRI and MRSI allow combined structural and metabolic evaluation of prostate cancer location, aggressiveness, and stage. Combined MRI and MRSI provide clinically and therapeutically relevant information that may assist in planning and post-treatment monitoring in patients undergoing radiation therapy.
INTRODUCTION
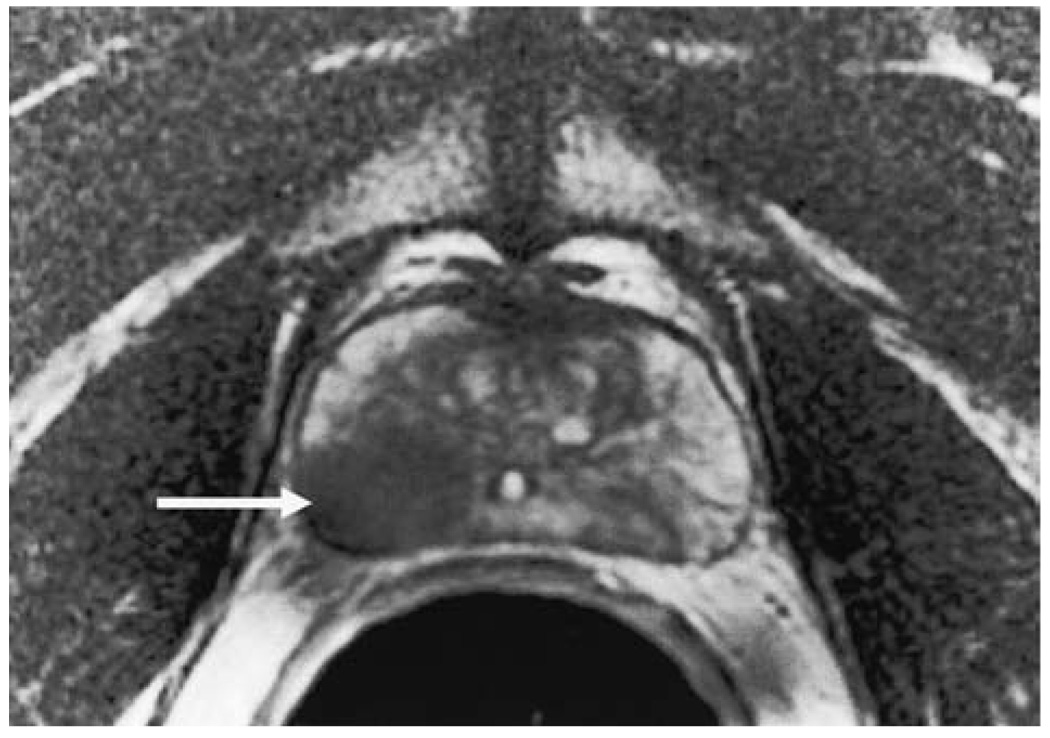
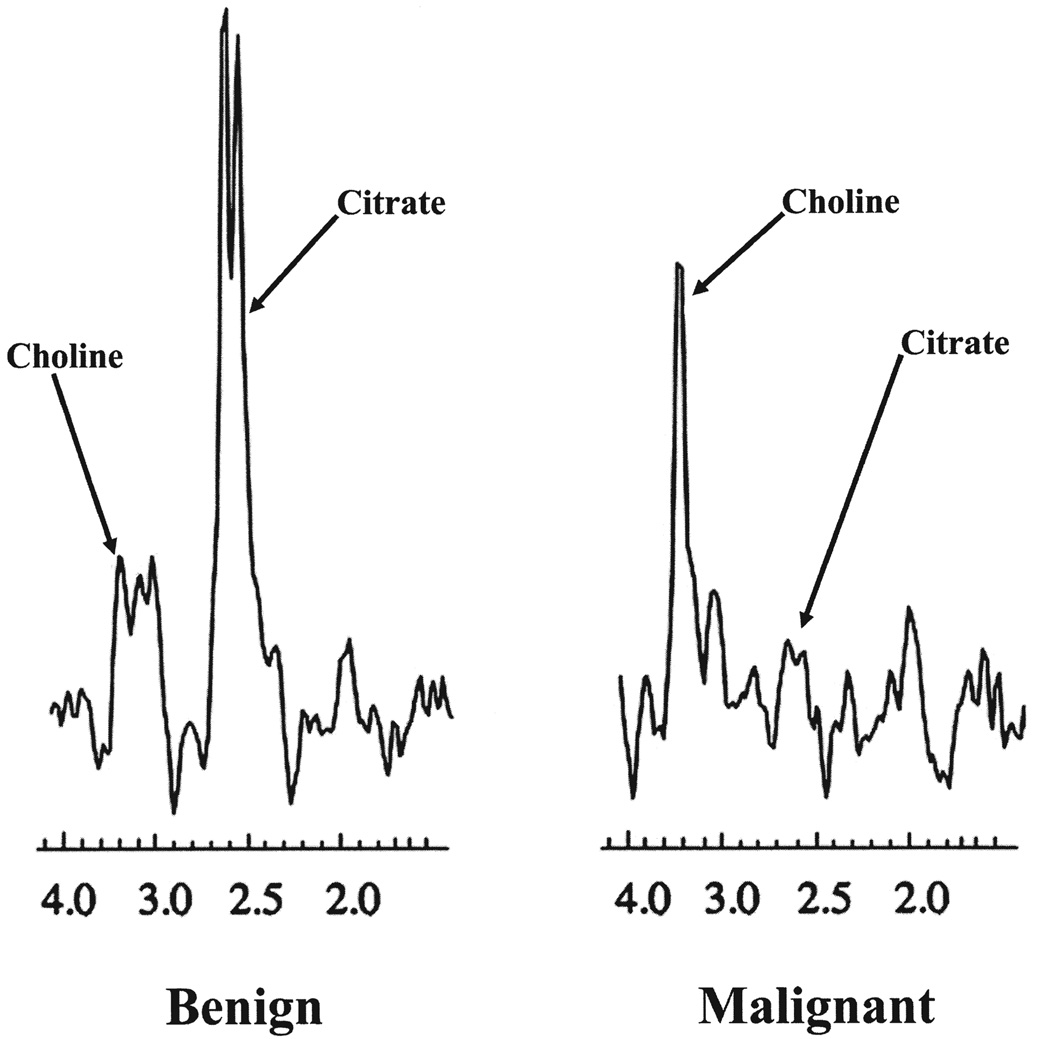
MAGNETIC RESONANCE IMAGING (MRI) uses strong magnetic fields to induce coherent spinning of hydrogen protons, and then applies radiofrequency pulses to generate a map of proton signal intensity by spatial location. Prostate cancer typically appears as a masslike or crescent subcapsular region of low T2 signal intensity in the peripheral zone of the prostate at magnetic resonance imaging (MRI)1 (Fig. 1). It should be noted that most publications related to MRI and magnetic resonance spectroscopic imaging (MRSI) of prostate cancer have focused on peripheral zone cancers, because these are the most common, and the characteristics of central gland tumors are less well established. During routine MRI, the signal intensity of all hydrogen protons is combined, although the signals from hydrogen protons in different molecules have slightly different frequencies (a property known as chemical shift). MRSI is an advanced form of MRI that exploits this chemical shift to produce a map of signal intensity versus frequency (i.e., a spectrum) that reflects the relative concentration of different metabolites. The metabolic peaks relevant to prostate MRSI are choline, creatine, and citrate. Research has shown that peripheral zone prostate cancer is characterized at MRSI by raised choline (a normal cell membrane constituent, which is elevated in many tumors) or reduced citrate (a constituent of normal prostatic tissue) or both2 (Fig. 2). Although complex interpretative schemes for prostate MRSI have been described,3 a simple approach that works reasonably well is to compare the height of the two metabolic peaks (i.e., choline plus creatine and citrate). If the first peak (choline plus creatine) is lower than the second (citrate), the voxel likely consists of normal tissue. On the other hand, the voxel likely consists of malignant tissue if the first peak is higher or of similar height to the second. Optimal results are obtained when both MRI and MRSI datasets are interpreted in an integrated fashion.4
FIG. 1.
Axial T2-weighted endorectal MR image of the prostate (TR 5000/TE 96) of a 57-year-old man with elevated PSA (8.1 ng/mL) and biopsy-proven cancer. A focal mass-like lesion of low signal intensity is identified within the peripheral zone (arrow).
FIG. 2.
Spectra of benign and malignant peripheral zone prostatic tissue. Note elevation of choline and decrease of citrate in cancer.
MRI and MRSI of the prostate can be performed as a single combined examination that takes about 60 minutes in total. An endorectal coil is essential for performance of spectroscopy, and, in addition, has been shown to improve the MRI component, resulting in higher staging accuracy.4a Special software is required for performance of MRSI. Several papers published in the last decade have described the role of MRI and MRSI before and after radiation therapy of prostate cancer, and therefore we undertook this review to describe the practical technical aspects of MRI and MRSI and to summarize the current and potential future status of MRI and MRSI in the localization, staging, treatment planning, and post-treatment follow-up of prostate cancer.
TECHNIQUE OF ENDORECTAL MRI AND MRSI OF THE PROSTATE
Prostate MRI protocols vary slightly among institutions, but in general will include at least the following: (1) T1-weighted images for detection of post-biopsy hemorrhage; (2) high-resolution T2-weighted images, in at least two different planes, usually axial and coronal; and (3) three-dimensional spectroscopic imaging of the entire gland (Table 1). Most institutions will perform the study using an endorectal coil in association with a pelvic-phased array coil. Although some authors contest the use of endorectal coil, especially after the advent of stronger magnets, it increases signal-to-noise ratio by a factor of 10, which results in higher spatial and spectral resolution.5–7 In fact, the use of an endorectal coil is essential for spectroscopic imaging. Multiple authors have compared the quality and accuracy of prostate MRI acquired using surface coils alone to those achievable with endorectal prostate MR imaging.4,8–11 Although the design and results of these studies differ, overall they show that the use of an endorectal coil is advantageous or at the very least provides results similar to an approach using a surface coil only.
Table 1.
Endorectal MRI/MRSI Protocol
| Sequence | Plane | TR/TE | Comments |
|---|---|---|---|
| Localizer | |||
| FSE | Sagittal | T2 | Evaluation of coil position. |
| SE | Axial | T1 | Entire pelvis, starting at the aortic bifurcation. Search for I post-biopsy hemorrhage. |
| FSE | Axial | T2 | Prostate evaluation. |
| FSE | Coronal | T2 | Prostate evaluation. |
| PROSE | Axial | Spectroscopy | Prostate evaluation. |
FSE = fast spin echo; SE = spin echo; PROSE = prostate spectroscopy and imaging examination.
THE ROLE OF MRI AND MRSI BEFORE RADIATION THERAPY
Prostate cancer is characterized by homogeneous low signal intensity on T2-weighted MR images against the normal high signal intensity of the peripheral zone.12 This finding, however, has limited sensitivity, because some tumors are isointense to the peripheral zone and there are benign causes of low signal intensity, such prostatitis, hemorrhage, and scarring.13–15 MRI is usually reserved for staging patients after a biopsy-proven diagnosis of prostate cancer, and only a few studies have investigated its use as a diagnostic tool. Some authors, however, have evaluated its use in patients with elevated prostate-specific antigen (PSA) and prior negative biopsies. The results of these studies suggest that MRI may be modestly useful as a tool to select patients and/or to provide guidance for further biopsy.16–18
MRI has no role as a screening tool for prostate cancer in the general population.
STAGING PROSTATE CANCER WITH MRI AND MRSI
Treatment of prostate cancer is greatly dependent on local staging, particularly the presence or absence of extracapsular extension. As a general rule, patients with disease localized to the prostate (stage T1 or T2 disease) are candidates for radical prostatectomy, whereas those with tumor extension beyond the prostatic capsule (stage T3 disease) are probably more appropriately managed with radiation therapy. Of course, those with low-risk disease, comorbidities, or limited life expectancy can be offered active surveillance.
Multiple studies have investigated the use of MRI for prostate cancer staging, with results showing an accuracy that reaches up to 95%, depending on research design, patient population, and age of technology.11,19–25 MRI seems to be most beneficial for the evaluation of moderate or high-risk patients with clinically localized disease. The findings that are most predictive of extracapsular extension on MRI are focal irregular capsular bulging, asymmetry of the neurovascular bundles, and obliteration of the rectoprostatic angle.26,27 The addition of MRSI to MRI appears to increase the accuracy of staging only for the less-experienced readers, but reduces interobserver variability.24,28
PREDICTIVE ROLE OF PRE-EBRT MRI
Preoperative endorectal MRI of the prostate has been investigated as a prognostic tool for predicting outcome after radical prostatectomy. D’Amico and his group published two studies evaluating the role of MRI to predict time to PSA failure after radical prostatectomy. The first study investigated the use of MRI in any men with clinically localized or PSA-detected prostate cancer,29 whereas the second targeted patients with clinically localized, but high-grade disease (biopsy Gleason score 7–10 and clinical T1c or T2a disease).30 According to their results, MRI provides relevant stratification of PSA outcome for these patients. Actuarial survival analysis showed that 22% and 28% of patients with stage T2 disease developed PSA failure after 3 and 5 years, respectively, versus 75% and 67% of patients who had stage T3 disease. More specifically, the detection of extracapsular extension significantly increased the risk of PSA failure. D’Amico has also shown that MRI was a better predictor of extracapsular extension, seminal vesicle invasion, and positive surgical margins than Gleason score or serum prostatic specific antigen level.31 Nonetheless, surgical series inevitably include patients with lower-risk tumors when compared to patients undergoing radiation therapy, and may not fully reflect the potential predictive value of MR findings.
Little is known regarding the predictive value of MRI prior to radiation therapy of prostate cancer. McKenna et al recently presented promising results showing that pretreatment endorectal MRI findings are predictive of outcome in patients who undergo external beam radiotherapy.32 Their data suggest that the presence and degree of extracapsular extension at MRI is a strong predictor of metastatic recurrence. Multivariate Cox analysis showed that the mean diameter of extracapsular extension was an independent predictive variable (relative hazard ratio 2.06, 95% confidence interval of 1.22–3.48). Although further investigation is needed, their results imply that patients with more than 5 mm of radiological extracapsular extension may be potential candidates for more aggressive therapy such as radiation dose escalation or extended androgen deprivation.
Nguyen et al published a paper in 2004 in which they demonstrated that the identification of seminal vesicle invasion on MRI in patients with clinically localized disease increased the risk of biochemical failure after external beam radiation therapy (EBRT).33 The risk of biochemical failure in that population was equivalent to the risk of failure in patients with locally advanced prostate cancer.
MRI AND RADIATION THERAPY PLANNING
Treatment of prostate cancer with EBRT has evolved. 3D conformal radiotherapy allows for an accurate delineation of the target organ, reducing the amount of radiation delivered to adjacent tissues. Intensity-modulated radiation therapy is a refinement of the 3D conformal radiation therapy. It refers to the technique that permits the modulation of each radiation beam, resulting in a conformal inhomogeneous field that maximizes irradiation dose to the tumor volume, while reducing the toxicity of radiation to the surrounding tissues.34 It is known that poor treatment coverage at radiotherapy is associated with increased risk of local and probably distant recurrence.35,36 The radial distance of extra prostatic extension of prostate cancer influences radiation treatment planning,37 because the dose falloff at the periphery of the field may result in incomplete treatment, although wide treatment margins increase the risk of radiation injury. T2-weighted MRI of the prostate has much higher soft tissue contrast than computed tomography (CT), which is usually used for planning EBRT. This high soft tissue contrast allows for the depiction of the zonal anatomy of the prostate, the extent of neoplastic disease, as well as the periprostatic structures.23,38–40 This leads to a more accurate delineation of the irradiation field, which decreases the dose to the periprostatic tissues and urethra, while delivering the maximum dosage to the target within the prostate.41–44
Similarly, an accurate identification and localization of prostate cancer is very important in patients who elect brachytherapy as primary or salvage treatment. Transrectal ultrasound imaging is routinely used as a guide for seed placement; however, an optimal distribution of radiation to a specific target is often impossible and in most cases, a maximum dosage will be delivered to the entire prostate. Because of its inherent high soft tissue contrast, MRI may overcome this limitation and guide targeted placement of brachytherapy seeds, concentrating the radiation dose in areas of larger tumor volume. Clarke et al found that MRI is a valuable tool for patients treated by seed implantation.45 It adequately identified cancer and modified clinical staging, permitting adequate coverage and increasing the success rate of treatment of prostate cancer. Seventy percent of the 327 patients in their study had an upgrade in staging after MRI, when compared to digital rectal examination-based clinical stage; 79 patients who were originally diagnosed with stage T1 and T2 disease were upstaged to T3. The proposed therapy was modified in 18% of their patients after evaluation of MRI findings. Furthermore, along with the percentage of positive core biopsies, the lack of a pretreatment MRI was a predictor of PSA failure in 38 months. Different MR-guided procedures have been developed, but those are not yet widely available.46–48
THE ROLE OF MRI AND MRSI AFTER RADIATION THERAPY
Follow-up after EBRT
Tumor detection by magnetic resonance imaging in the irradiated gland is limited by treatment changes that include prostatic shrinkage, the development of diffuse low T2 signal intensity in the gland, and indistinctness of the normal zonal anatomy.49–51 MRSI, which detects abnormal metabolism rather than abnormal anatomy, has shown considerable promise in the local evaluation of prostate cancer prior to treatment.24,52,53 Preliminary studies evaluating MRSI in the local evaluation of prostate cancer after radiation therapy have also been promising.54–56 Coakley et al, examining 21 patients with biochemical failure after EBRT, found that MRSI had an area under the receiver-operating curve of 81% for the detection of recurrent cancer.54 Endorectal MRI alone performed poorly in this study, with an area under the receiver-operating curve (AU-ROC) of 49–50%. In a similar study, Pucar et al found that MRSI had an AU-ROC of 88% for discrimination of benign and malignant prostatic tissue after EBRT.55 In the study by Menard et al, the sensitivity and specificity of MRSI for diagnosing post-treatment malignancy were 88.9% and 92%, respectively, with an accuracy of 91.4%.56 The results of these studies suggest that MRSI has the ability to detect or exclude local recurrence within the prostate and could facilitate salvage treatment, or potentially support the decision to commence systemic therapy in patients with presumed distant disease based on biochemical failure in the absence of detectable local recurrence
In another study, Pickett et al demonstrated that post-treatment MRI and MRSI increase the level of confidence of local control assessment, helping to distinguish a benign PSA blip from local recurrence.57
Follow-up after brachytherapy
In patients who undergo brachytherapy, the adequacy of dosimetry largely depends on accurate quantification of the prostate volume after the implantation of seeds. The most common method used for this purpose is CT.58 However, several authors have demonstrated the improvement of volume determination with MRI when compared to CT. Although CT overestimates the volume of the gland,59–61 MRI decreases the interobserver variability in prostate contouring62, 63 and allows better identification of the gland apex.44,64,65 MRI may also be the tool of choice to follow up these patients for treatment response and to assess locoregional tissue toxicity. Coakley et al, for instance, have demonstrated that MRI clearly depicts both intra- and extraprostatic seed distribution, while evaluating treatment changes.66
CONCLUSION
Prostate MRI/MRSI is a valuable staging procedure for patients with prostate cancer. Furthermore, it has the ability to predict PSA outcome after radiation therapy in intermediate and high-risk patients, acting as an additional guide for adequate treatment selection. Because of its high soft tissue contrast, MRI provides better delineation of the tumor and periprostatic structures than CT does. This leads to a more accurate demarcation of the irradiation field, decreasing treatment-related toxicity. Finally, MRI/MRSI is an adequate tool to follow up patients treated with radiation therapy. It clearly depicts the expected changes related to treatment, while allowing for the detection of persistent or recurrent disease.
ACKNOWLEDGMENT
A.C.W. was supported by NIBIB T32 Training Grant 1 T32 EB001631-01A1.
REFERENCES
- 1.Rajesh A, Coakley FV. MR imaging and MR spectroscopic imaging of prostate cancer. Magn Reson Imaging Clin N Am. 2004;123:557. doi: 10.1016/j.mric.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Kurhanewicz J, Vigneron DB, Hricak H, Narayan P, Carroll P, Nelson SJ. Three-dimensional H-1 MR spectroscopic imaging of the in situ human prostate with high (0.24-0.7-cm3) spatial resolution. Radiology. 1996;1983:795. doi: 10.1148/radiology.198.3.8628874. [DOI] [PubMed] [Google Scholar]
- 3.Jung JA, Coakley FV, Vigneron DB, et al. Prostate depiction at endorectal MR spectroscopic imaging: Investigation of a standardized evaluation system. Radiology. 2004;2333:701. doi: 10.1148/radiol.2333030672. [DOI] [PubMed] [Google Scholar]
- 4.Westphalen AC, Coakley FV, Qayyum A, et al. Peripheral zone prostate cancer: Accuracy of different interpretative approaches with MR and MR spectroscopic imaging. Radiology. 2008;246:177–184. doi: 10.1148/radiol.2453062042. [DOI] [PubMed] [Google Scholar]
- 4a.Hricak H, White S, Vigneron D, et al. Carcinoma of the prostate gland: MR imaging with pelvic phased-array coils versus integrated endorectal—pelvic phased-array coils. Radiology. 1994;1933:703. doi: 10.1148/radiology.193.3.7972810. [DOI] [PubMed] [Google Scholar]
- 5.Bloch BN, Rofsky NM, Baroni RH, Marquis RP, Pedrosa I, Lenkinski RE. 3 Tesla magnetic resonance imaging of the prostate with combined pelvic phased-array and endorectal coils: Initial experience. Acad Radiol. 2004;118:863. doi: 10.1016/j.acra.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 6.Chen AP, Cunningham CH, Kurhanewicz J, et al. High-resolution 3D MR spectroscopic imaging of the prostate at 3 T with the MLEV-PRESS sequence. Magn Reson Imaging. 2006;247:825. doi: 10.1016/j.mri.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Futterer JJ, Scheenen TW, Huisman HJ, et al. Initial experience of 3 tesla endorectal coil magnetic resonance imaging and 1H-spectroscopic imaging of the prostate. Invest Radiol. 2004;3911:671. doi: 10.1097/00004424-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Beyersdorff D, Taymoorian K, Knosel T, et al. MRI of prostate cancer at 1.5 and 3.0 T: Comparison of image quality in tumor detection and staging. AJR Am J Roentgenol. 2005;1855:1214. doi: 10.2214/AJR.04.1584. [DOI] [PubMed] [Google Scholar]
- 9.Kaji Y, Wada A, Imaoka I, et al. Proton two-dimensional chemical shift imaging for evaluation of prostate cancer: External surface coil vs. endorectal surface coil. J Magn Reson Imaging. 2002;166:697. doi: 10.1002/jmri.10204. [DOI] [PubMed] [Google Scholar]
- 10.Sosna J, Pedrosa I, Dewolf WC, Mahallati H, Lenkinski RE, Rofsky NM. MR imaging of the prostate at 3 Tesla: Comparison of an external phased-array coil to imaging with an endorectal coil at 1.5 Tesla. Acad Radiol. 2004;118:857. doi: 10.1016/j.acra.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Futterer JJ, Engelbrecht MR, Jager GJ, et al. Prostate cancer: Comparison of local staging accuracy of pelvic phased-array coil alone versus integrated endorectal-pelvic phased-array coils. Local staging accuracy of prostate cancer using endorectal coil MR imaging. Eur Radiol. 2007;174:1055. doi: 10.1007/s00330-006-0418-8. [DOI] [PubMed] [Google Scholar]
- 12.Bezzi M, Kressel HY, Allen KS, et al. Prostatic carcinoma: Staging with MR imaging at 1.5 T. Radiology. 1988;1692:339. doi: 10.1148/radiology.169.2.3174982. [DOI] [PubMed] [Google Scholar]
- 13.Lovett K, Rifkin MD, McCue PA, Choi H. MR imaging characteristics of noncancerous lesions of the prostate. J Magn Reson Imaging. 1992;21:35. doi: 10.1002/jmri.1880020106. [DOI] [PubMed] [Google Scholar]
- 14.Quint LE, Van Erp JS, Bland PH, et al. Prostate cancer: Correlation of MR images with tissue optical density at pathologic examination. Radiology. 1991;1793:837. doi: 10.1148/radiology.179.3.2028002. [DOI] [PubMed] [Google Scholar]
- 15.White S, Hricak H, Forstner R, et al. Prostate cancer: Effect of postbiopsy hemorrhage on interpretation of MR images. Radiology. 1995;1952:385. doi: 10.1148/radiology.195.2.7724756. [DOI] [PubMed] [Google Scholar]
- 16.Perrotti M, Han KR, Epstein RE, et al. Prospective evaluation of endorectal magnetic resonance imaging to detect tumor foci in men with prior negative prostastic biopsy: A pilot study. J Urol. 1999;1624:1314. [PubMed] [Google Scholar]
- 17.Beyersdorff D, Taupitz M, Winkelmann B, et al. Patients with a history of elevated prostate-specific antigen levels and negative transrectal US-guided quadrant or sextant biopsy results: Value of MR imaging. Radiology. 2002;2243:701. doi: 10.1148/radiol.2243011553. [DOI] [PubMed] [Google Scholar]
- 18.Costouros NG, Coakley FV, Westphalen AC, et al. Diagnosis of prostate cancer in patients with an elevated prostate-specific antigen level: Role of endorectal MRI and MR spectroscopic imaging. Am J Roentgenol. 2007;1883:812. doi: 10.2214/AJR.06.0165. [DOI] [PubMed] [Google Scholar]
- 19.Heijmink SW, Futterer JJ, Hambrock T, et al. Prostate cancer: Body-array versus endorectal coil MR imaging at 3 T—comparison of image quality, localization, and staging performance. Radiology. 2007;2441:184. doi: 10.1148/radiol.2441060425. [DOI] [PubMed] [Google Scholar]
- 20.Chandra RV, Heinze S, Dowling R, Shadbolt C, Costello A, Pedersen J. Endorectal magnetic resonance imaging staging of prostate cancer. ANZ J Surg. 2007;7710:860. doi: 10.1111/j.1445-2197.2007.04259.x. [DOI] [PubMed] [Google Scholar]
- 21.Park BK, Kim B, Kim CK, Lee HM, Kwon GY. Comparison of phased-array 3.0-T and endorectal 1.5-T magnetic resonance imaging in the evaluation of local staging accuracy for prostate cancer. J Comput Assist Tomogr. 2007;314:534. doi: 10.1097/01.rct.0000250108.85799.e1. [DOI] [PubMed] [Google Scholar]
- 22.Brassell SA, Krueger WR, Choi JH, Taylor JA., 3rd Correlation of endorectal coil magnetic resonance imaging of the prostate with pathologic stage. World J Urol. 2004;224:289. doi: 10.1007/s00345-004-0440-x. [DOI] [PubMed] [Google Scholar]
- 23.Akin O, Sala E, Moskowitz CS, et al. Transition zone prostate cancers: Features, detection, localization, and staging at endorectal MR imaging. Radiology. 2006;2393:784. doi: 10.1148/radiol.2392050949. [DOI] [PubMed] [Google Scholar]
- 24.Yu KK, Scheidler J, Hricak H, et al. Prostate cancer: Prediction of extracapsular extension with endorectal MR imaging and three-dimensional proton MR spectroscopic imaging. Radiology. 1999;2132:481. doi: 10.1148/radiology.213.2.r99nv26481. [DOI] [PubMed] [Google Scholar]
- 25.Huch Boni RA, Boner JA, Debatin JF, et al. Optimization of prostate carcinoma staging: Comparison of imaging and clinical methods. Clin Radiol. 1995;509:593. doi: 10.1016/s0009-9260(05)83287-8. [DOI] [PubMed] [Google Scholar]
- 26.Outwater EK, Petersen RO, Siegelman ES, Gomella LG, Chernesky CE, Mitchell DG. Prostate carcinoma: Assessment of diagnostic criteria for capsular penetration on endorectal coil MR images. Radiology. 1994;1932:333. doi: 10.1148/radiology.193.2.7972739. [DOI] [PubMed] [Google Scholar]
- 27.Yu KK, Hricak H, Alagappan R, Chernoff DM, Bacchetti P, Zaloudek CJ. Detection of extracapsular extension of prostate carcinoma with endorectal and phased-array coil MR imaging: Multivariate feature analysis. Radiology. 1997;2023:697. doi: 10.1148/radiology.202.3.9051019. [DOI] [PubMed] [Google Scholar]
- 28.Wetter A, Ajdukovic AN, Fliessbach K, et al. Staging of prostate cancer: Value of the combined information of endorectal MRI, biopsy Gleason score, and preoperative PSA level. [German] Rofo. 2006;178:385–390. doi: 10.1055/s-2006-926475. [DOI] [PubMed] [Google Scholar]
- 29.D’Amico AV, Whittington R, Malkowicz B, et al. Endorectal magnetic resonance imaging as a predictor of biochemical outcome after radical prostatectomy in men with clinically localized prostate cancer. J Urol. 2000;164:759. doi: 10.1097/00005392-200009010-00032. [DOI] [PubMed] [Google Scholar]
- 30.Cheng GC, Chen MH, Whittington R, et al. Clinical utility of endorectal MRI in determining PSA outcome for patients with biopsy Gleason score 7, PSA <or=10, and clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2003;551:64. doi: 10.1016/s0360-3016(02)03820-8. [DOI] [PubMed] [Google Scholar]
- 31.D’Amico AV, Whittington R, Malkowicz SB, et al. Critical analysis of the ability of the endorectal coil magnetic resonance imaging scan to predict pathologic stage, margin status, and postoperative prostate-specific antigen failure in patients with clinically organ-confined prostate cancer. J Clin Oncol. 1996;146:1770. doi: 10.1200/JCO.1996.14.6.1770. [DOI] [PubMed] [Google Scholar]
- 32.McKeena DA, Westphalen AC, Qayyum A, Roach M, Kurhanewicz J, Coakley FV. Pre-treatment magnetic resonance imaging features of prostate cancer as a predictor of response to external beam radiotherapy. AJR. 2007;1885:A31. doi: 10.1016/j.ijrobp.2008.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen PL, Whittington R, Koo S, et al. Quantifying the impact of seminal vesicle invasion identified using endorectal magnetic resonance imaging on PSA outcome after radiation therapy for patients with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2004;592:400. doi: 10.1016/j.ijrobp.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 34.Zelefsky MJ, Fuks Z, Hunt M, et al. High-dose intensity modulated radiation therapy for prostate cancer: Early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys. 2002;535:1111. doi: 10.1016/s0360-3016(02)02857-2. [DOI] [PubMed] [Google Scholar]
- 35.Yorke ED, Fuks Z, Norton L, Whitmore W, Ling CC. Modeling the development of metastases from primary and locally recurrent tumors: Comparison with a clinical data base for prostatic cancer. Cancer Res. 1993;5313:2987. [PubMed] [Google Scholar]
- 36.Fuks Z, Leibel SA, Wallner KE, et al. The effect of local control on metastatic dissemination in carcinoma of the prostate: Longterm results in patients treated with 125I implantation. Int J Radiat Oncol Biol Phys. 1991;213:537. doi: 10.1016/0360-3016(91)90668-t. [DOI] [PubMed] [Google Scholar]
- 37.Davis BJ, Pisansky TM, Wilson TM, et al. The radial distance of extraprostatic extension of prostate carcinoma: Implications for prostate brachytherapy. Cancer. 1999;8512:2630. [PubMed] [Google Scholar]
- 38.Graser A, Heuck A, Sommer B, et al. Per-sextant localization and staging of prostate cancer: Correlation of imaging findings with whole-mount step section histopathology. AJR Am J Roentgenol. 2007;1881:84. doi: 10.2214/AJR.06.0401. [DOI] [PubMed] [Google Scholar]
- 39.Mullerad M, Hricak H, Kuroiwa K, et al. Comparison of endorectal magnetic resonance imaging, guided prostate biopsy and digital rectal examination in the preoperative anatomical localization of prostate cancer. J Urol. 2005;1746:2158. doi: 10.1097/01.ju.0000181224.95276.82. [DOI] [PubMed] [Google Scholar]
- 40.Sala E, Eberhardt SC, Akin O, et al. Endorectal MR imaging before salvage prostatectomy: Tumor localization and staging. Radiology. 2006;2381:176. doi: 10.1148/radiol.2381052345. [DOI] [PubMed] [Google Scholar]
- 41.Albert M, Tempany CM, Schultz D, et al. Late genitourinary and gastrointestinal toxicity after magnetic resonance image-guided prostate brachytherapy with or without neoadjuvant external beam radiation therapy. Cancer. 2003;985:949. doi: 10.1002/cncr.11595. [DOI] [PubMed] [Google Scholar]
- 42.De Meerleer G, Villeirs G, Bral S, et al. The magnetic resonance detected intraprostatic lesion in prostate cancer: Planning and delivery of intensity-modulated radiotherapy. Radiother Oncol. 2005;753:325. doi: 10.1016/j.radonc.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 43.Khoo VS, Padhani AR, Tanner SF, Finnigan DJ, Leach MO, Dearnaley DP. Comparison of MRI with CT for the radiotherapy planning of prostate cancer: A feasibility study. Br J Radiol. 1999;72858:590. doi: 10.1259/bjr.72.858.10560342. [DOI] [PubMed] [Google Scholar]
- 44.Debois M, Oyen R, Maes F, et al. The contribution of magnetic resonance imaging to the three-dimensional treatment planning of localized prostate cancer. Int J Radiat Oncol Biol Phys. 1999;454:857. doi: 10.1016/s0360-3016(99)00288-6. [DOI] [PubMed] [Google Scholar]
- 45.Clarke DH, Banks SJ, Wiederhorn AR, et al. The role of endorectal coil MRI in patient selection and treatment planning for prostate seed implants. Int J Radiat Oncol Biol Phys. 2002;524:903. doi: 10.1016/s0360-3016(01)02736-5. [DOI] [PubMed] [Google Scholar]
- 46.Barnes AS, Haker SJ, Mulkern RV, So D, D’Amico AV, Tempany CM. Magnetic resonance spectroscopy-guided transperineal prostate biopsy and brachytherapy for recurrent prostate cancer. Urology. 2005;666:1319. doi: 10.1016/j.urology.2005.06.105. [DOI] [PubMed] [Google Scholar]
- 47.Susil RC, Camphausen K, Choyke P, et al. System for prostate brachytherapy and biopsy in a standard 1.5 T MRI scanner. Magn Reson Med. 2004;523:683. doi: 10.1002/mrm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zaider M, Zelefsky MJ, Lee EK, et al. Treatment planning for prostate implants using magnetic-resonance spectroscopy imaging. Int J Radiat Oncol Biol Phys. 2000;474:1085. doi: 10.1016/s0360-3016(00)00557-5. [DOI] [PubMed] [Google Scholar]
- 49.Chan TW, Kressel HY. Prostate and seminal vesicles after irradiation: MR appearance. J Magn Reson Imaging. 1991;15:503. doi: 10.1002/jmri.1880010502. [DOI] [PubMed] [Google Scholar]
- 50.Coakley FV, Hricak H, Wefer AE, Speight JL, Kurhanewicz J, Roach M. Brachytherapy for prostate cancer: Endorectal MR imaging of local treatment-related changes. Radiology. 2001;2193:817. doi: 10.1148/radiology.219.3.r01jn46817. [DOI] [PubMed] [Google Scholar]
- 51.Coakley FV, Teh HS, Qayyum A, et al. Endorectal MR imaging and MR spectroscopic imaging for locally recurrent prostate cancer after external beam radiation therapy: Preliminary experience. Radiology. 2004;2332:441. doi: 10.1148/radiol.2332032086. [DOI] [PubMed] [Google Scholar]
- 52.Coakley FV, Kurhanewicz J, Lu Y, et al. Prostate cancer tumor volume: Measurement with endorectal MR and MR spectroscopic imaging. Radiology. 2002;2231:91. doi: 10.1148/radiol.2231010575. [DOI] [PubMed] [Google Scholar]
- 53.Scheidler J, Hricak H, Vigneron DB, et al. Prostate cancer: Localization with three-dimensional proton MR spectroscopic imaging—clinicopathologic study. Radiology. 1999;2132:473. doi: 10.1148/radiology.213.2.r99nv23473. [DOI] [PubMed] [Google Scholar]
- 54.Coakley FV, Teh HS, Qayyum A, et al. Endorectal MR imaging and MR spectroscopic imaging for locally recurrent prostate cancer after external beam radiation therapy: Preliminary experience. Radiology. 2004;233:441–448. doi: 10.1148/radiol.2332032086. [DOI] [PubMed] [Google Scholar]
- 55.Pucar D, Shukla-Dave A, Hricak H, et al. Prostate cancer: Correlation of MR imaging and MR spectroscopy with pathologic findings after radiation therapy—initial experience. Radiology. 2005;2362:545. doi: 10.1148/radiol.2362040739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Menard C, Smith IC, Somorjai RL, et al. Magnetic resonance spectroscopy of the malignant prostate gland after radiotherapy: A histopathologic study of diagnostic validity. Int J Radiat Oncol Biol Phys. 2001;502:317. doi: 10.1016/s0360-3016(01)01480-8. [DOI] [PubMed] [Google Scholar]
- 57.Pickett B, Kurhanewicz J, Coakley F, Shinohara K, Fein B, Roach M. Use of MRI and spectroscopy in evaluation of external beam radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2004;604:1047. doi: 10.1016/j.ijrobp.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 58.Nag S, Bice W, DeWyngaert K, Prestidge B, Stock R, Yu Y. The American Brachytherapy Society recommendations for permanent prostate brachytherapy postimplant dosimetric analysis. Int J Radiat Oncol Biol Phys. 2000;461:221. doi: 10.1016/s0360-3016(99)00351-x. [DOI] [PubMed] [Google Scholar]
- 59.Roach M, 3rd, Faillace-Akazawa P, Malfatti C, Holland J, Hricak H. Prostate volumes defined by magnetic resonance imaging and computerized tomographic scans for three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 1996;355:1011. doi: 10.1016/0360-3016(96)00232-5. [DOI] [PubMed] [Google Scholar]
- 60.Rasch C, Barillot I, Remeijer P, Touw A, van Herk M, Lebesque JV. Definition of the prostate in CT and MRI: A multi-observer study. Int J Radiat Oncol Biol Phys. 1999;431:57. doi: 10.1016/s0360-3016(98)00351-4. [DOI] [PubMed] [Google Scholar]
- 61.McLaughlin PW, Narayana V, Drake DG, et al. Comparison of MRI pulse sequences in defining prostate volume after permanent implantation. Int J Radiat Oncol Biol Phys. 2002;543:703. doi: 10.1016/s0360-3016(02)02991-7. [DOI] [PubMed] [Google Scholar]
- 62.Parker CC, Damyanovich A, Haycocks T, Haider M, Bayley A, Catton CN. Magnetic resonance imaging in the radiation treatment planning of localized prostate cancer using intra-prostatic fiducial markers for computed tomography co-registration. Radiother Oncol. 2003;662:217. doi: 10.1016/s0167-8140(02)00407-3. [DOI] [PubMed] [Google Scholar]
- 63.Smith WL, Lewis C, Bauman G, et al. Prostate volume contouring: A 3D analysis of segmentation using 3DTRUS, CT, and MR. Int J Radiat Oncol Biol Phys. 2007;674:1238. doi: 10.1016/j.ijrobp.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 64.Milosevic M, Voruganti S, Blend R, et al. Magnetic resonance imaging (MRI) for localization of the prostatic apex: Comparison to computed tomography (CT) and urethrography. Radiother Oncol. 1998;473:277. doi: 10.1016/s0167-8140(97)00232-6. [DOI] [PubMed] [Google Scholar]
- 65.Algan O, Hanks GE, Shaer AH. Localization of the prostatic apex for radiation treatment planning. Int J Radiat Oncol Biol Phys. 1995;334:925. doi: 10.1016/0360-3016(95)00226-4. [DOI] [PubMed] [Google Scholar]
- 66.Coakley FV, Hricak H, Wefer AE, Speight JL, Kurhanewicz J, Roach M., 3rd Brachytherapy for prostate cancer: Endorectal MR imaging of local treatment-related changes. Radiology. 2001;2193:817. doi: 10.1148/radiology.219.3.r01jn46817. [DOI] [PubMed] [Google Scholar]