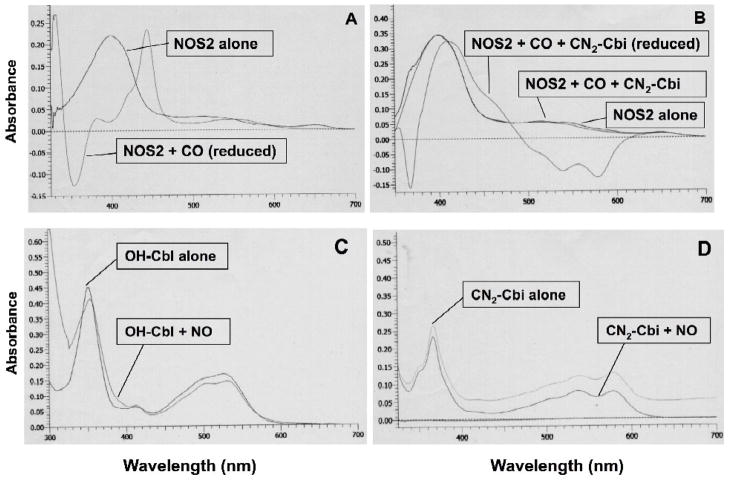
Figure 5. A–D. Spectroscopy of dicyanocobinamide interactions with purified, recombinant NOS2 oxygenase domain, or NO.
Dicyanocobinamide reacts directly with purified, recombinant NOS2 oxygenase domain (A & B): Oxygen reacts directly with the reduced iron of the heme group in NOS. We used carbon monoxide (CO) (that simulates O2 in binding to the reduced iron) to determine the influence of CN2-Cbi on oxygen binding to NOS. The reaction of purified NOS2 oxygenase domain (approximately 3 μM final concentration) with CO in a reduced state (with dithionite) markedly changed the UV/vis spectrum, with appearance of the typical CO-bound Fe+2-heme peak at 445 nm (A). However, in the presence of CN2-Cbi, there was no appearance of the 445 nm peak induced by CO in a reduced state using NOS2 oxygenase domain at a final concentration of approximately 4.5 μM (B). Similar findings were noted with OH-Cbl instead of CN2-Cbi.
Hydroxocobalamin binds NO, but dicyanocobinamide does not (C & D). NO reacts with OH-Cbl (C) as indicated by the change in the UV/Vis spectrum (shift of peak from 352 nm to 354–355 nm, and alteration in the 520 nm region). However, NO does not react with CN2-Cbi (D) (no change in spectrum).