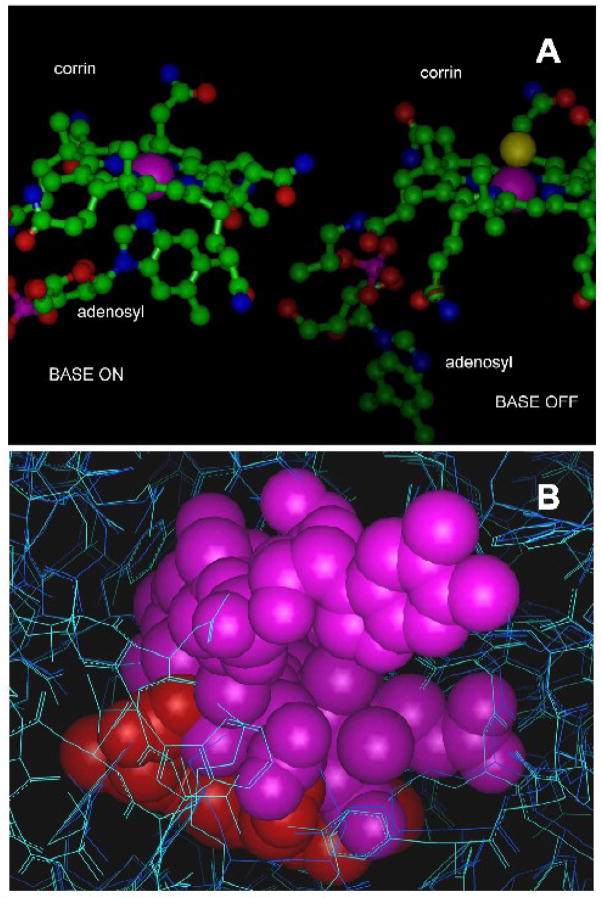
Figure 6. Structural studies of cobalamin-NOS interactions.
6A. Structures of adenosylcobalamin in the “base-on” and “base-off” configurations showing the positions of the corrin ring and the dimethylbenzimidazole moiety. The purple spheres represent the Van der Waals surface of the cobalt atom. The structure of the “base-off” form was taken from the structure of glutamate mutase reported by Reitzer et al (PDB entry 1cb7) [44]. In this structure, the cobalt ligand trans to the methyl axial ligand is a histidyl residue (not shown) supplied by the protein. The “base-on” form shown was taken from the structure of cobalamin bound to transcobalamin reported by Wuerges et al (PDB entry 2bb5) [45]. The axial ligand trans to dimethylbenzimidazole is again a histidyl residue (not shown) supplied by the protein. Slight differences in the planarity of the corrins include movement of the cobalt towards dimethylbenzimidazole in the “base-on” configuration. Considerable flexibility in the molecule exists, making many other orientations of the base possible.
6B. Docking of the “base-off” form of cobalamin (as in Figure 5A) in the heme pockets of NOS2 (dark blue) and NOS1 (cyan), illustrating the extent of the ligand-binding cavity relative to the size of cobalamin. Heme is shown in red and cobalamin in purple. The very large substrate-binding pocket accommodates surprisingly large ligands, including porphyrins (similar in size and shape to the corrin shown here). The insertion of cobalamin into the heme pocket is ultimately limited by a beta sheet near V567 (NOS1). The configuration shown generates a 0.4Angstrom clash with the valine side chain in the crystal structure from PDB P_1ZVL, and represents the approximate limit of insertion of cobalamin, assuming slight rearrangements in the backbone are possible. A similar configuration with approximately 80% overlap of the corrin and heme can be obtained without backbone or unrelaxable side chain clashes. Slight torsioning of some active site residue side chains and repositioning of the DMBzI moiety of the cobalamin (visible as rings in solid rendering at the upper right) was necessary. The aromatic side chains visible at the bottom of the figure at the edge of the access channel are W306 and Y706 in NOS1.