Figure 2.
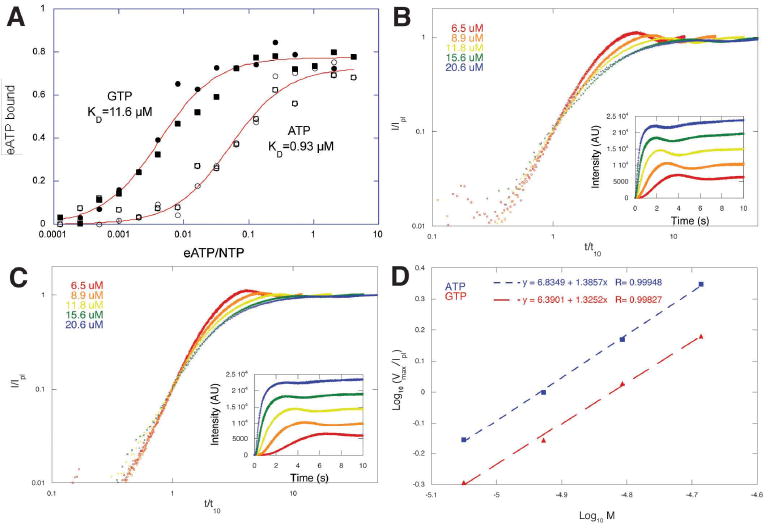
ParM accomodates ATP and GTP. (A) Competition of etheno-ATP away from ParM by ATP and GTP. The affinity of ParM for ATP (Kd=0.9 μM) is more than 10-fold higher than for GTP (Kd=11.6 μM). The experiment was performed three times with identical results. Conditions: Buffer: 100 mM KCl, 1 mM MgCl2, 10 mM Hepes, pH 7.0. Temperature: 24°C. (B) Assembly of five different concentrations of R1 ParM initiated by addition of 5 mM ATP. (C) Assembly of the same concentrations of ParM initiated by addition of 5 mM GTP. Polymerization was monitored by right angle light scattering and the amplitude of the light scattering signal normalized to the value at plateau. Time scale were normalized to the time required to reach 10% of the plateau value. Insets: raw data before normalization. The similarity of the slopes of the curves at early time points indicates that, in each case, the nucleation mechanism is the same at all concentrations tested. (D) Log-log plot of the normalized maximum velocity of polymerization versus ParM concentration. The identical slopes argue that the mechanism of nucleation is the same in both cases. The offset between the lines suggests that spontaneous nucleation is sligthly faster in the presence of ATP.
