Fig. 6.
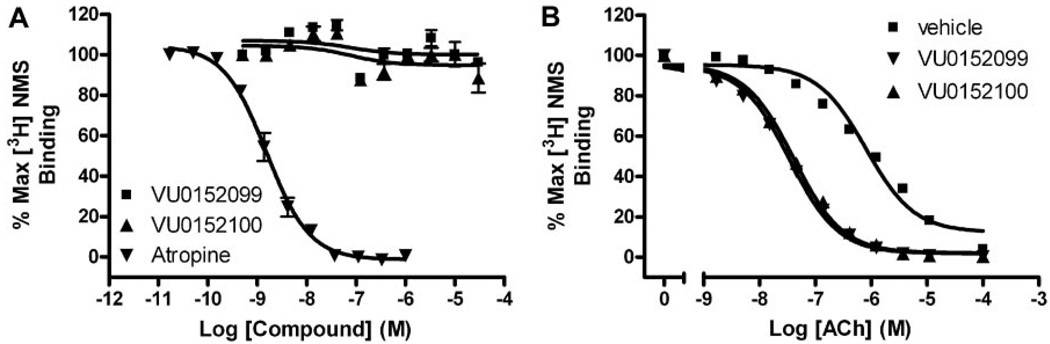
VU0152099 and VU0152100 bind allosterically and increase ACh affinity at rM4. A, in competition binding studies, neither VU0152099 (■) nor VU0152100 (▲) displaced the orthosteric radioligand, [3H]NMS (0.1 nM), at concentrations up to 30 µM. However, the orthosteric antagonist, atropine (▲), potently inhibited [3H]NMS binding with a Ki of 0.54 ± 0.1 nM. B, in the presence of vehicle alone, an increasing concentration of ACh displaces [3H]NMS (0.1 nM) binding with a Ki of 252 ± 17.9 nM (■). In the presence of a fixed concentration (10 µM) of VU0152099 or VU0152100, the potency of ACh to displace [3H]NMS binding is shifted leftward, yielding Ki values of 10.4 ± 0.91 (▼, VU0152099) and 12.2 ± 0.49 nM (▲,VU0152100), which represent a 25- and 21-fold shift in ACh potency, respectively. Data represent the mean ± S.E.M. of three independent experiments performed in duplicate.