Abstract
Medial artery vascular smooth muscle cell (VSMC) calcification increases the risk of cardiovascular mortality in type 2 diabetes. However, the influence of insulin on VSMC calcification is unclear. We explored the effects of insulin on rat VSMC calcification in vitro and found that in a dose-dependent fashion, insulin attenuates VSMC calcification induced by high phosphate conditions as quantified by the OCPC method. In an in vitro model of insulin resistance in which cells are exposed to elevated insulin concentrations and the PI 3-kinase pathway is selectively inhibited, increased VSMC calcification was observed, suggesting that the PI 3-kinase pathway is involved in this attenuating effect of insulin. We postulated that insulin may also have an effect on phosphate or calcium transport in VSMC. We found that insulin increases phosphate transport at 3 hr and 24 hr. This effect was mediated by increased Vmax for phosphate transport but not Km. Because type III sodium-phosphate co-transporters Pit-1 and Pit-2 are found in VSMC, we examined their expression by Western blot and real-time RT-PCR. Insulin stimulates Pit-1 mRNA modestly (*p<0.01 vs. control), an effect mediated by PD98059 but not by wortmannin. Pit-1 protein expression is induced by insulin, an effect also mediated by PD98059 (*p<0.001 vs. insulin alone). Results for Pit-2 were mixed. Our results suggest a role for insulin in attenuating VSMC calcification which may be disrupted in selective insulin signaling impairment seen in insulin resistance. This effect of insulin contrasts with its effect to induce phosphate transport in VSMC.
Keywords: vascular smooth muscle (rat), insulin, phosphate transport, intracellular signaling, calcification
INTRODUCTION
Medial artery calcification is an independent predictor of cardiovascular mortality in individuals with type 2 diabetes mellitus (1–3) and end-stage renal disease (4). The mechanisms for vascular calcification in these clinical settings are still being elucidated (5,6), but increased vascular calcification is clearly associated with hyperphosphatemia (7) and with increased serum calcium-phosphate ion product.
A key feature of type 2 diabetes is insulin resistance. In the early stages of the development of type 2 diabetes, pancreatic beta-cell insulin secretion increases to compensate for the decreased action of insulin in target tissues such as muscle, adipose and liver. Insulin per se and the compensatory hyperinsulinemia of insulin resistance exert important actions on the vascular wall (8–10). For example, insulin induces nitric oxide synthase expression and augments its activity in endothelial cells under physiologic conditions, increasing nitric oxide production and resulting in vasodilation (10). Insulin also promotes and maintains differentiation of vascular smooth muscle cells (9). These anti-atherogenic effects of insulin in endothelial and vascular smooth muscle cells are mediated via phosphatidylinositol (PI) 3-kinase-dependent signaling (8,9), which is selectively impaired in insulin resistant states. On the other hand, pro-atherogenic effects of hyperinsulinemia mediated through extracellular regulated kinase (ERK) 1/2 mitogen activated protein (MAP) kinase pathway-dependent signaling are unaffected in insulin resistance, and may even be augmented (8,9).
Contribution of either insulin resistance or hyperinsulinemia to vascular calcification remains poorly understood. Insulin seems to have mixed effects on calcium flux, with some investigators demonstrating attenuation of inward calcium currents in rat VSMC in response to arginine vasopressin (11) and angiotensin II (12), and others showing stimulation of calcium uptake (13). In this study, we examined the effect of insulin on calcification of rat VSMC, and on VSMC phosphate and calcium transport. We used pharmacologic inhibitors of PI 3-kinase and ERK 1/2 MAP kinase-dependent signaling to determine mechanisms of insulin action on these processes.
MATERIALS
Rat vascular smooth muscle cells (VSMC) were generously provided by Drs. Nihal Kaplan-Albuquerque and Raphael Nemenoff at the University of Colorado Health Sciences Center, Denver, Colorado. Cell culture media and reagents were from Invitrogen (Carlsbad, CA). Calcification was quantified with an OCPC kit (Wako Diagnostics, Richmond, VA). Lactate dehydrogenase (LDH) enzyme activity was assayed using a Cytotoxicity Detection Kit (LDH) (Roche Molecular Biochemicals, Mannheim, Germany). LY294002 and U0126 were from Cell Signaling Technology (Beverly, MA) and PD98059 from Calbiochem (San Diego, CA). 32PO4 was obtained from Perkin Elmer (Boston, MA) as orthophosphoric acid. Specific type III sodium-phosphate co-transporter (Pit-1 and Pit-2) antibodies were from Alpha Diagnostic International (San Antonio, TX). Protein was quantified using the bicinchoninic acid (BCA) protein assay method (Pierce, Rockford, IL). ECL Plus kits were from Amersham (Piscataway, NJ). For RNA isolation and processing, Trizol and RNase-free DNase (Invitrogen) were used. The iScript cDNA Synthesis kit for reverse transcription and iQ Sybr Green Supermix kit for real time PCR were from Bio-Rad (Hercules, CA). Primers were synthesized by Integrated DNA Technologies (Coralville, IA). All other reagents and supplies were from Sigma (St. Louis, MO) and Fisher (Hampton, NH). GraphPad Prism software (San Diego, CA) was used for all statistical and kinetic analyses.
METHODS
Cell culture
Rat VSMC were passaged in flasks after enzymatic digestion of adult rat thoracic aortas. The culture method and growth medium have been previously described (9). The growth medium does not contain insulin; relevant molecules include calcium (1.8 mM), phosphate (1.014 mM), and glucose (5.6 mM). VSMC primary cultures were used up to the ninth passage (14). All cells were placed in serum-reduced medium (≤0.2% serum) for 24 hr to maintain quiescence prior to further treatment.
Measurement of phosphate transport activity and kinetics
Since we postulated that insulin might exert an effect on phosphate transport, we etermined whether insulin affected VSMC phosphate transport. After reaching subconfluence, VSMC were grown in 6-well plates and incubated in serum-reduced medium for 24 hr, then with dimethylsulfoxide (DMSO, 1:1000 vol/vol, control) or insulin 10 nM, in the presence or absence of wortmannin 100 nM or PD98059 20 μM for 3 hr or 24 hr. Just prior to uptake experiments, the VSMC monolayers were rinsed at 37°C with transport solution containing 137 mM NaCl, 5.4 mM KCl, 2.8 mM CaCl2, 1.2 mM MgSO4, and 14 mM HEPES, pH 7.4 (15). To measure total sodium- phosphate (Na/Pi) co-transport, VSMC were incubated with 500 μl of transport solution also containing 0.05 mM K2H32PO4. To measure Na-independent phosphate (Pi) uptake, sodium chloride (NaCl) was substituted equimolarly with choline chloride in the washing and uptake solutions. We performed transport kinetic studies by measuring Na/Pi co-transport activity in the presence of 0.01 to 5 mM extracellular Pi at 6 min of incubation. After 6 minutes, within the initial rate of linear uptake, transport was terminated by aspiration of the uptake solution and washing the monolayers thrice at 4°C. The cells were then lysed in 0.5% Triton X-100 and analyzed for 32PO4 counts. Protein concentration was determined using the Pierce BCA protein assay method. Transport activity is expressed as pmol Pi/mg protein/min.
To determine whether the effects of insulin modulation on Na/Pi co-transport activity were mediated by alterations in the capacity or maximal velocity (Vmax) of the co-transporter, or by the affinity for Pi (Km), we performed transport kinetic studies by measuring Na/Pi co-transport activity in the presence of 0.01 to 5 mM extracellular Pi. An equation containing saturable (Michaelis-Menten, or transport) and non-saturable (diffusion) components (16) was used to calculate the Km, Vmax and Kd coefficients by iterative, non-linear regression: V = [Vmax · S/(Km + S)] + (Kd · S). The first term of the equation involves saturable uptake exclusively, i.e. transport. The second term refers to the non-saturable uptake, and involves diffusion through the plasma membrane and intercellular junctions as well as experimental unspecific binding. The fits were accepted when two consecutive iterations changed the sum of squares by less than 0.01%. The significance of the differences among the fits was calculated with an F-test, using the sum-of-squares and degrees of freedom from the fits. The significances of the differences between kinetic constants were obtained with a t-test, with p<0.05 considered statistically significant.
Calcium uptake in VSMC
Calcium levels in VSMC were measured according to the work of Giuliano and Wood (17) with some modifications. Rather than fluxes, we measured the steady-state concentration of 45CaCl2 in VSMC after 30 min of incubation in the original transport buffer consisting of 140 mM NaCl, 5.8 mM KCl, 0.34 mM Na2HPO4, 0.8 mM MgSO4, 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 4 mM glutamine, and 25 mM glucose, pH 7.4. Otherwise, the method was identical to that used in determining phosphate transport activity and kinetics.
Western blotting
Rat VSMC were grown to subconfluence, then incubated with insulin 10 nM for 24 hr. Cells were lysed and sonicated, and protein quantified using the Pierce BCA assay system. Aliquots of whole lysates were dried in a Speedvac Concentrator (Savant, Holbrook, NY), resuspended in Laemmli sample buffer, and stored at −20°C until used. Samples were boiled briefly, loaded for SDS-polyacrylamide gel electrophoresis, and transferred to polyvinylidene fluoride membranes. Membranes were probed with rabbit polyclonal antibodies specific for type III sodium-phosphate co-transporter 1 or 2 (Pit-1 or Pit-2, Alpha Diagnostic), then incubated with goat anti-rabbit polyclonal antibodies conjugated to horseradish peroxidase. Pit-1 or Pit-2 protein was detected using an ECL Plus kit, and quantitative densitometric analysis was performed on a Bio-Rad Fluor-S MultiImager. Intracellular signaling pathways were examined by incubating cells with wortmannin 100 nM, PD98059 20 μM, or U0126 20 μM, in the presence or absence of insulin.
Quantification of Pit-1 and Pit-2 mRNA expression
The abundance of sodium-phosphate (Na/Pi) co-transporter transcripts was determined by real-time polymerase chain reaction (PCR). Rat VSMC were grown to subconfluence, then incubated with insulin 10 nM for 3 hr or 24 hr. Total RNA from VSMC monolayers was isolated with Trizol. One μg RNA per sample was then digested with amplification grade RNase free DNase, and reverse transcribed with an iScript cDNA Synthesis kit following manufacturer’s instructions. Specific amplification and quantification of Pit-1 (121 bp length, accession number NM031148) and Pit-2 (126 bp length, accession number NM017223) transcripts was done using an iQ Sybr Green Supermix kit in an iCycler iQ Real-Time PCR Detection System (Bio-Rad). The comparative CT method was used for quantification, with acidic ribosomal phosphoprotein RNA (Arp, 147 bp length, accession number NM022402) as an endogenous reference. Primers were designed using Beacon Designer software (Premier Biosoft International, Palo Alto, CA) with the indicated accession numbers as template sequences:
Pit-1 Upper: 5′-CCGTCAGCAACCAGATCAACTC-3′
Pit-1 Lower: 5′-CCCATGCAGTCTCCCACCTTG-3′
Pit-2 Upper: 5′-CTATTCCAAGAAGAGGCTCCG-3′
Pit-2 Lower: 5′-TCAGGATCGGTCAGCTCAG-3′
Arp Upper: 5′-CACCTTCCCACTGGCTGAA-3′
Arp Lower: 5′-TCCTCCGACTCTTCCTTTGC-3′
Intracellular signaling pathways were examined by incubating VSMC with insulin 10 nM in the absence or presence of wortmannin 100 nM or PD98059 20 μM.
Quantitative and qualitative determination of VSMC calcification
Rat VSMC were grown to subconfluence, then switched to serum-reduced medium for 24 hr. Cells were subsequently incubated in serum-reduced medium containing either normal phosphate (1.014 mM) or high phosphate (2 mM). VSMC were then treated with insulin 10 nM in the presence or absence of intracellular signaling pathway inhibitors wortmannin 100 nM, PD98059 20 μM, or U0126 20 μM. Media and treatments were changed every 24 hr. After cells in the high phosphate medium-treated group had calcified, cells were washed with 0.9% NaCl and decalcified with 0.6 N HCl overnight at 4°C. Plates were then scraped to obtain VSMC lysates. Protein was quantified using the BCA protein assay, and the OCPC method of color development with o-cresolphthalein was used to quantify calcium content (in μg/mg protein).
In a separate set of experiments, an insulin dose-response curve was constructed for VSMC calcification, in which cells were prepared as described above, then treated with either 25 mM HEPES buffer as control, or insulin in doses ranging from 0.1 nM to 10 nM. Serum-reduced high phosphate medium, control buffer and insulin was changed every 24 hr until cells in the control wells had calcified. VSMC were then processed as described above for calcium quantitation by OCPC method.
For qualitative staining using alizarin red, VSMC were grown to subconfluence, then switched to serum-reduced medium and treated as above. Once cells in the high phosphate medium-treated group had calcified, VSMC were rinsed with water, plates were drained, and 2% alizarin red solution (pH 4.1) was added. After 30 sec incubation at room temperature, the plates were rinsed three times with water, and visualized with the 10x objective (total magnification 100×) of a Zeiss Axiovert 40 microscope using brightfield illumination and an Axiocam HR camera.
LDH assay for total cell lysis
To address whether increased VSMC calcification was due to certain treatments or from increased cell death, cytotoxicity was determined by measuring LDH enzymatic activity of cell supernatants. Rat VSMC were cultured and serum-starved as described above. Cells were then incubated in serum-reduced medium along with either DMSO (control), wortmannin 100 nM, or PD98059 20 μM, or switched to high-phosphate (2 mM phosphate) serum-reduced medium containing either insulin 10 nM or insulin 10 nM plus wortmannin 100 mM for an additional 24 hr. VSMC supernatants were then collected, centrifuged to remove cell debris, and kept at ≤−20°C until further analysis. A Cytotoxicity Detection Kit (LDH) from Roche Molecular Biochemicals was used to assay for LDH enzymatic activity, since this correlates with increased numbers of dead or plasma membrane-damaged cells resulting from lysis of the cells. The results are expressed as the percentage of total LDH activity obtained by lysing the cells with 1% Triton X-100 in the same assay medium.
Determination of Caspase-3 activity
To further address the question of whether increased cell death was responsible for the increased VSMC calcification observed with particular treatments, caspase-3 activity, an early marker of apoptotic cell death, was examined. Rat VSMC were cultured and serum-starved as described above, then incubated in serum-reduced medium along with either DMSO (control), wortmannin 100 nM, or PD98059 20 μM, or switched to high-phosphate (2 mM phosphate) serum-reduced medium containing either insulin 10 nM or insulin 10 nM plus wortmannin 100 mM for an additional 24 hr. VSMC were then trypsinized and centrifuged, and the supernatant discarded. Cell pellets were kept at ≤−20°C until further analysis. A Caspase-3 Assay Kit by Sigma (St. Louis, MO) was then used to measure caspase-3 activity. The assay is based on caspase-3 hydrolysis of a peptide substrate (acetyl-Asp-Glu-Val-Asp p-nitroanilide) which results in release of a p-nitroaniline moiety. This is detected spectrophotometrically at 405 nm, and the concentration of p-nitroaniline released from the sample is calculated from this information. Caspase-3 activity is proportional to p-nitroaniline concentration.
Trypan blue exclusion
Rat VSMC were cultured and serum-starved as described above, then incubated in serum-reduced medium along with either DMSO (control), wortmannin 100 nM, or PD98059 20 μM, or switched to high-phosphate (2 mM phosphate) serum-reduced medium containing either insulin 10 nM or insulin 10 nM plus wortmannin 100 mM for an additional 24 hr. Cells were lifted with trypsin-EDTA, spun down, and cell pellets resuspended in 1 × PBS. Trypan blue 0.4% suspension was added. After 5 minutes and before 15 minutes of incubation, cells excluding (white) or not excluding (blue) trypan blue dye were counted using a hemacytometer. Five fields were counted for each treatment, and 4 experiments were performed for each treatment group. Percentage viability of the cells was determined by calculating the number of white cells divided by the total number (blue + white), and multiplying by 100%.
Statistical Analyses
Results are expressed as mean±SEM of between three and six independent experiments that were compared using a paired or unpaired t-test or ANOVA as indicated. P values of <0.05 were considered statistically significant. As mentioned in the Methods, the significance of differences among fits for the effects of insulin on Na/Pi co-transport activity was calculated with an F-test, using the sum-of-squares and degrees of freedom from the fits. The significances of the differences between kinetic constants were obtained with a t-test, with p<0.05 considered statistically significant. Calculations with ANOVA showed the same degree of significance.
EXPERIMENTAL RESULTS
Insulin protects against VSMC calcification
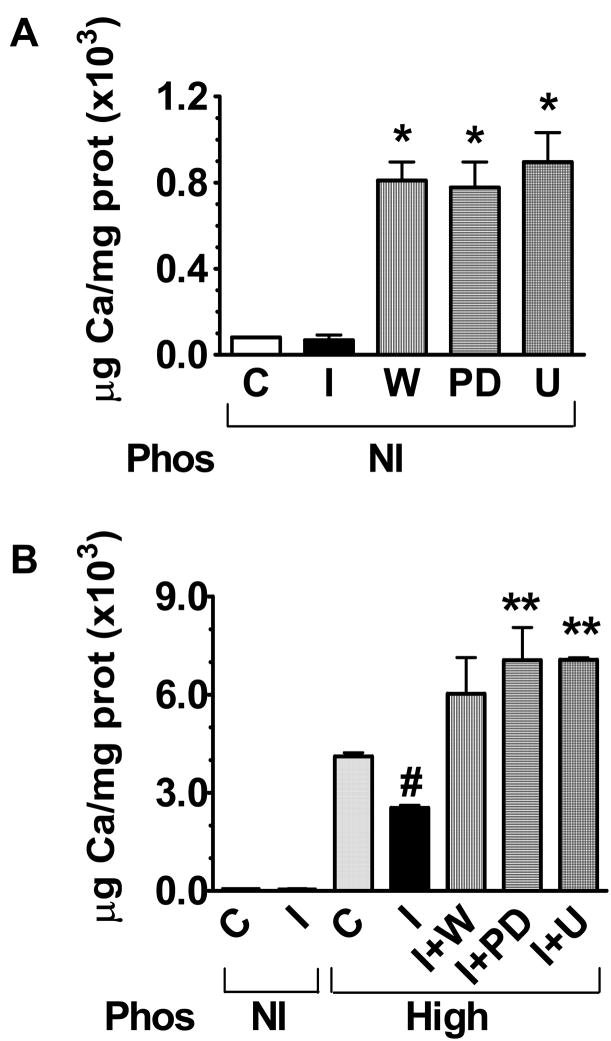
Since medial calcification increases the risk for cardiovascular disease in type 2 diabetes, we examined whether insulin itself has an effect on VSMC calcification. To address this, rat VSMC were treated with insulin in serum-reduced normal (1.014 mM) or high (2 mM) phosphate medium, and the effects on calcification were quantified in mg Ca/mg protein. Incubation of VSMC with insulin 10 nM in normal phosphate medium did not influence the VSMC calcification process after 8–10 days of incubation compared with control (Fig. 1A,B; Fig. 2A). The presence of high phosphate (2 mM) in the medium resulted in increased VSMC calcification compared with normal phosphate, as expected (Fig. 1C; Fig. 2B). Insulin attenuated VSMC calcification induced by high phosphate conditions (Fig. 1D; 2B, p<0.05).
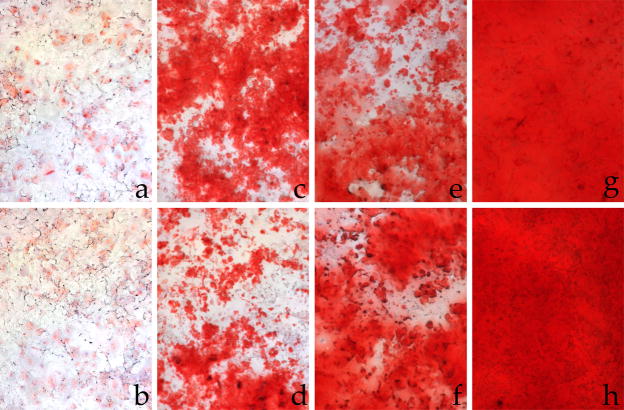
Figure 1.
Qualitative alizarin red staining for calcium in rat VSMC. Incubation in normal phosphate (1.014 mM) medium with A. DMSO or B. insulin, in high (2 mM) phosphate medium with C. DMSO, D. insulin, E. wortmannin, F. PD98059, G. insulin with wortmannin, or H. insulin with PD98059 for 8–10 days. A 10x objective was used under brightfield illumination for all panels (total magnification 100×). Insulin attenuates high phosphate-induced calcification but not signaling pathway inhibition-induced calcification.
Figure 2.
VSMC calcification by OCPC method (see Methods) after incubation with DMSO (control, C) or insulin (I), with or without wortmannin (W), PD98059 (PD), or U0126 (U), in either normal phosphate (1.014 mM) or high phosphate (2 mM, Pi) medium for 8–10 days(n=5). A. Insulin alone in normal phosphate medium did not affect VSMC calcification. Wortmannin and PD98059 each markedly increased VSMC calcification (*p<0.05 vs. I). B. High phosphate medium increased VSMC calcification markedly while insulin attenuated this (#p<0.05 vs. Pi). Insulin was unable to protect against calcification induced by high phosphate medium in the presence of wortmannin, PD98059 or U0126 (**p<0.05 vs. Pi+I).
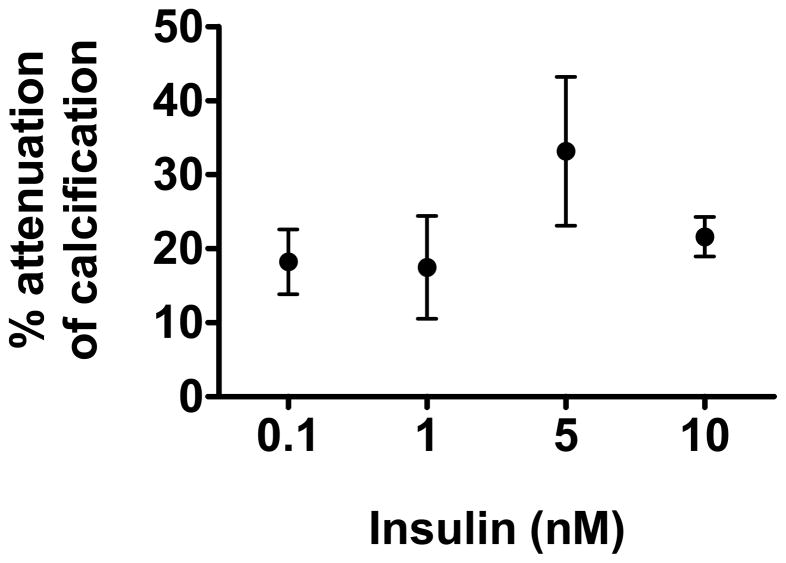
We performed a dose-response curve for insulin effects on VSMC calcification and found that insulin at concentrations as low as 0.1 nM and 1 nM attenuates VSMC calcification by approximately 20%. Insulin attenuates VSMC calcification maximally at 5 nM (by 33%), and its effect continues to be significant at 10 nM (Fig. 3).
Figure 3.
Insulin dose-response for VSMC calcification in high phosphate (2 mM, Pi) medium for 8–10 days (n=3) by OCPC assay. Results are expressed as %attenuation of calcification. Control VSMC were incubated in 25 mM HEPES buffer, while dose response consisted of cells incubated in 0.1 nM, 1 nM, 5 nM or 10 nM insulin. Maximal attenuation of calcification was achieved with 5 nM insulin (*p<0.05 vs. 5 nM or 10 nM insulin compared with control).
Since there is selective impairment of the PI 3-kinase signaling pathway as well as compensatory hyperinsulinemia in vitro, in rodent models of insulin resistance, and in humans (18–20), we exposed rat VSMC to elevated insulin concentrations while selectively inhibiting the PI 3-kinase pathway with wortmannin 100 nM. In our in vitro model of insulin resistance, insulin lost its ability to attenuate VSMC calcification (Fig. 1G; 2B). To find out if the other major intracellular signaling pathway activated by insulin, the ERK 1/2 MAP kinase pathway, plays a role in insulin attenuation of VSMC calcification, we selectively inhibited the ERK 1/2 MAP kinase pathway with PD98059 20 μM (Fig. 1H; Fig. 2B) or U0126 20 μM (Fig. 2B) under high phosphate conditions. We found that inhibiting this pathway also causes insulin to lose its attenuating effect on VSMC calcification, suggesting a role for ERK 1/2 MAP kinase signaling in VSMC calcification.
The increased VSMC calcification observed was not due to increased cell lysis
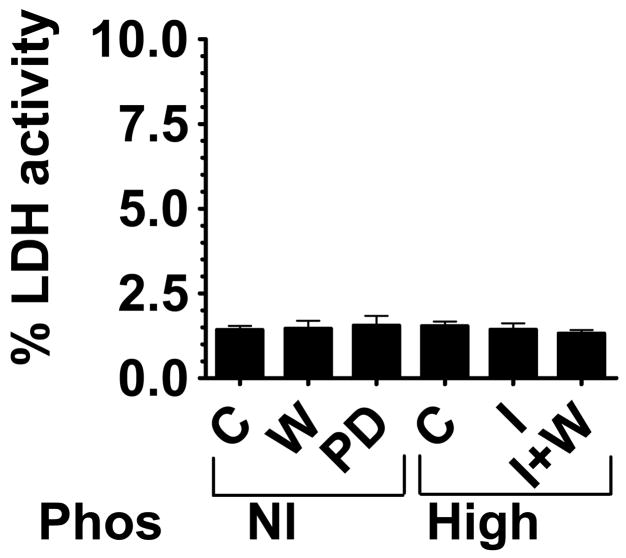
To determine whether the increased VSMC calcification was simply due to increased cell lysis or cell death, three methods were employed: lactate dehydrogenase (LDH) enzymatic activity, caspase-3 activity, and trypan blue exclusion. LDH and caspase-3 activity should increase if there is increased cell lysis or cell death from any cause, and cell death eliminates the ability of a cell to exclude trypan blue dye. We found that LDH activity was unchanged among the different treatment groups (Fig. 4). Caspase 3 activity and trypan blue exclusion confirmed these results (not shown), with no differences found among treatment groups.
Figure 4.
Percentage LDH enzyme activity as a measure of cytotoxicity. VSMC supernatants were assayed for LDH enzyme activity as a measure of cell death after incubation with DMSO as a control (C), wortmannin (W), PD98059 (PD), or in high (2mM) phosphate medium with (Pi+I) or without insulin (Pi) or insulin and wortmannin (Pi+I+W). The control treated cells had 1.42% ±0.12 cytotoxicity. All other treatment groups were not significantly different from control.
Insulin stimulates VSMC phosphate transport by increasing Vmax, but does not affect calcium transport
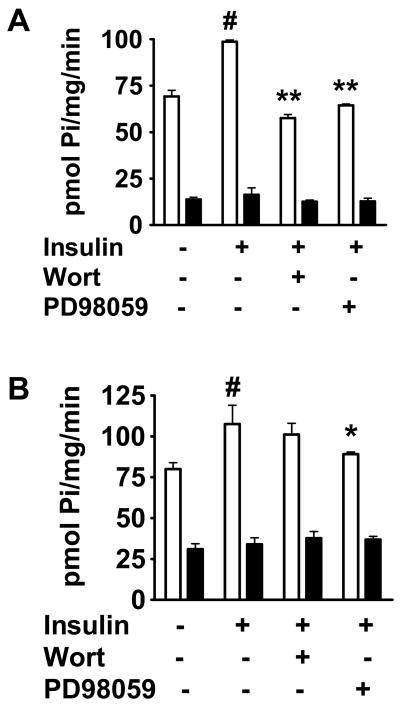
To determine whether insulin attenuation of VSMC calcification is correlated with changes in V\SMC phosphate transport, we examined whether insulin affects inorganic phosphate (Pi) transport in VSMC. Rat VSMC were incubated for either 3 hr or 24 hr with insulin 10 nM or with dimethylsulfoxide (DMSO) as control. As early as 3 hr, insulin caused a 1.4-fold increase (p<0.01) in sodium-dependent phosphate transport compared with control (Fig. 5A). This effect of insulin was maintained at 24 hr (Fig. 5B, p<0.05).
Figure 5.
Effect of insulin on phosphate transport (n=3). Cells were incubated with DMSO (control) or insulin, with or without wortmannin or PD98059. A. After 3 hr, insulin stimulates sodium-dependent phosphate transport to 1.4-fold control (#p<0.01). This is inhibited by wortmannin and PD98059 (**p<0.0001 vs. insulin for each). B. After 24 hr, insulin stimulates sodium-dependent phosphate transport to 1.5-fold control (#p<0.05). This is inhibited only by PD98059 (*p<0.05 vs. insulin). White bars = uptake in presence of sodium chloride, black bars = uptake in presence of choline chloride.
Insulin activates two major intracellular signaling pathways: the PI 3-kinase and ERK 1/2 MAP kinase pathways, so we examined the relative contributions of these pathways to the mechanism of insulin action on VSMC phosphate transport. Rat VSMC were incubated for either 3 hr or 24 hr with insulin in the presence of either wortmannin 100 nM, a PI 3-kinase pathway inhibitor, or PD98059 20 μM, an ERK 1/2 MAP kinase pathway inhibitor. Inhibition of the ERK 1/2 MAP kinase pathway abolished insulin’s stimulatory effect on phosphate transport in VSMC at 3 hr and at 24 hr (Fig. 5A,B). PI 3-kinase pathway inhibition impaired the effect of insulin at 3 hr (Fig. 5A) but its influence was not statistically significant by 24 hr (Fig. 5B). None of the inhibitors alone had any effect on phosphate transport (not shown).
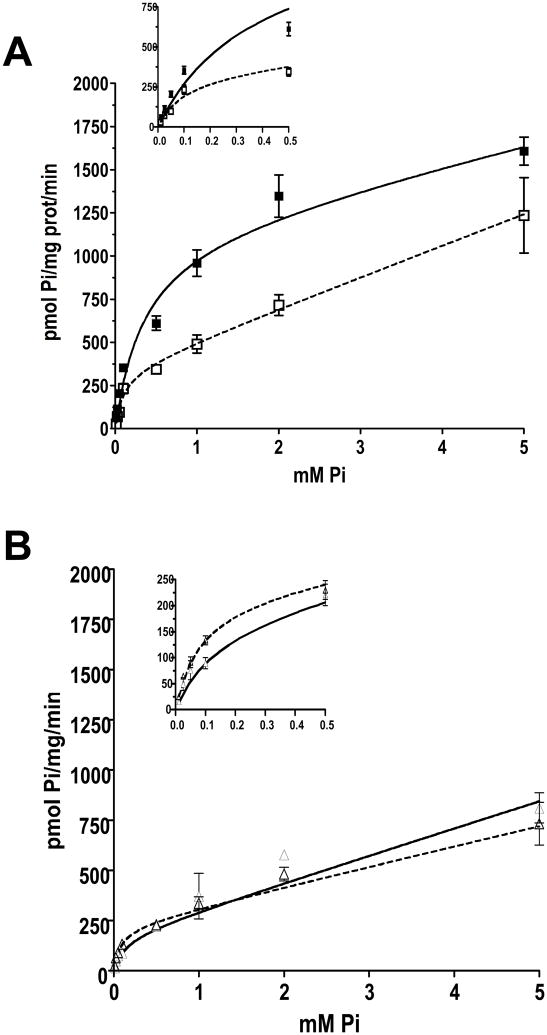
To determine the effects of 3 hr treatment with 10 nM insulin on the kinetic characteristics of phosphate transport systems, michaelian-saturation uptake assays were performed. The data were fit to an equation containing a saturation component plus a non-saturable, diffusional member (see Methods). In the presence of NaCl, control vs. insulin treatment fits were significantly different (F=25.142, p<0.0001). This difference was caused by a 100% increase in the capacity (Vmax) of phosphate transport in insulin treated cells (Fig. 6A, Table 1). This effect was strictly dependent on the presence of Na+, because the Vmax and Km of insulin-stimulated sodium-independent phosphate transport in VSMC was not different from control (Fig. 6B, Table 1; F test 0.545, p = 0.6541).
Figure 6.
Insulin effects on phosphate transport kinetics in VSMC in the presence of A. sodium chloride (inset shows the points at lower Pi concentrations) or B. choline chloride (n=3). Sodium-phosphate co-transport was measured in response to 0 to 5 mM extracellular phosphate (Pi). Insulin increases Vmax but not Km (p<0.05 vs. control). Black squares, solid line = insulin; white squares, dotted line = control.
TABLE 1.
Sodium-dependent and -independent phosphate transport kinetics in rat VSMC
| NaCl |
Choline Cl |
|||
|---|---|---|---|---|
| Control | Insulin | Control | Insulin | |
| Vmax(pmol Pi/mg/min) | 353.2 ± 93.30 | 852.6 ± 84.70* | 220.2 ± 26.90 | 176.6 ± 61.50 |
| Km(mM) | 0.115 ± 0.039 | 0.171 ± 0.023 | 0.079 ± 0.015 | 0.133 ± 0.059 |
| Kd (pI/mg/min) | 178.8 ± 60.90 | 167.3 ± 27.60 | 100.9 ± 14.69 | 134.6 ± 33.79 |
| r | 0.9099 | 0.9640 | 0.9174 | 0.8898 |
Data are expressed as mean ± SEM.
p<0.05
The effect of insulin on calcium transport was also examined in VSMC. Insulin had no measurable influence on 45Ca2+ incorporation at the end of the 30 min uptake assay (not shown).
Insulin effects on Pit-1 and Pit-2 in VSMC
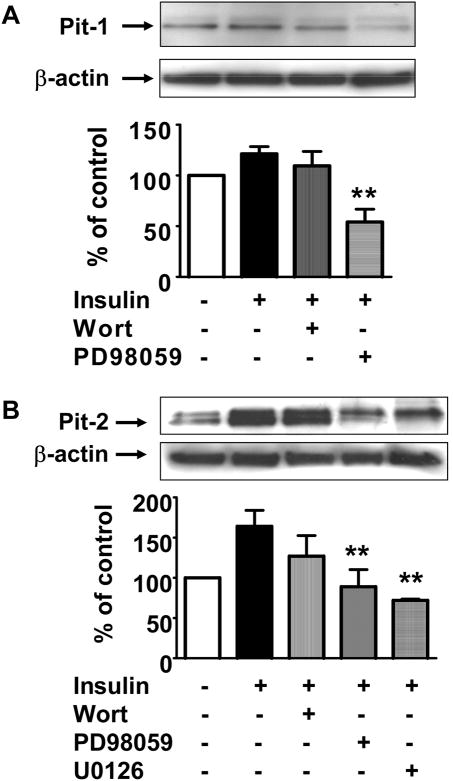
Type III sodium-phosphate (Na/Pi) co-transporters (Pit-1 and Pit-2) are expressed in VSMC primary cultures and mediate phosphate transport. To assess whether the effect of insulin on phosphate transport is due to increased phosphate transporter expression, protein and mRNA expression of these transporters were examined in the presence or absence of insulin 10 nM. At 24 hr, insulin induced a small but significant increase in Pit-1 protein (Fig. 7A, 121.3±7.1% of control, p<0.05). The addition of wortmannin 100 nM did not affect insulin effect on Pit-1 protein. However, ERK 1/2 MAP kinase pathway inhibition with PD98059 20 μM significantly decreased VSMC Pit-1 protein expression (Fig. 7A, p<0.001 vs. insulin).
Figure 7.
Pit-1 and Pit-2 protein expression by Western blot after 24 hr incubation with DMSO (control) or insulin, with or without wortmannin, PD98059, or U0126 (n=6). Representative Western blots showing Pit-1 and Pit-2 bands with β-actin (loading control) above corresponding bars in graph below. A. Insulin increases Pit-1 protein slightly (21%). Wortmannin had no effect, but PD98059 reduced Pit-1 protein expression (**p<0.001 vs. insulin). B. Insulin increased Pit-2 protein to 164% control. However, PD98059 and U0126 in the presence of insulin decreased Pit-2 protein significantly (**p<0.05 compared with insulin). Wortmannin had no effect.
Insulin caused a 1.6-fold increase in Pit-2 protein at 24 hr (Fig. 7B, 164.1±19.9% of control, p<0.05). This was not affected significantly by inhibition of the PI 3-kinase pathway with wortmannin. However, ERK 1/2 MAP kinase pathway inhibition with either PD98059 or U0126 20 μM significantly blocked this effect of insulin on Pit-2 protein expression (p<0.05 vs. insulin). None of the inhibitors alone had any effect on Pit-1 or Pit-2 protein expression (not shown).
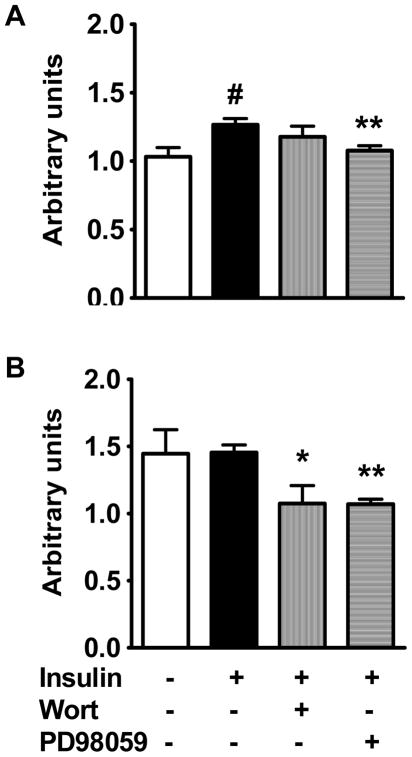
Real-time RT-PCR was used to evaluate mRNA expression. Insulin does not affect Pit-1 mRNA expression at 3 hr (not shown) although it caused a slight increase in Pit-1 mRNA expression compared with control at 24 hr (Fig. 8A, p<0.01). This effect of insulin at 24 hr was reduced by PD98059 (p<0.01) but not by wortmannin. Pit-2 mRNA was not affected significantly by insulin at 3 hr or 24 hr. However, at 24 hr, inhibition of the PI 3-kinase pathway with wortmannin or the ERK 1/2 MAP kinase pathway with PD98059 significantly decreased Pit-2 mRNA expression (p<0.05 and p<0.01 respectively vs. insulin, Fig. 8B).
Figure 8.
Pit-1 and Pit-2 mRNA expression by real-time RT-PCR after 24 hr incubation with DMSO (control) or insulin, with or without wortmannin or PD98059 (n=4). A. Insulin stimulated Pit-1 mRNA to 1.2-fold control (#p<0.01). This was inhibited by PD98059 (**p<0.01 vs. insulin) but not wortmannin. B. Insulin did not affect Pit-2 mRNA, but wortmannin and PD98059 each significantly decreased Pit-2 expression (*p<0.05 and **p<0.01 vs. insulin respectively).
DISCUSSION
The salient observation of this study is that insulin attenuates VSMC calcification induced by high phosphate conditions. In an in vitro model of insulin resistance in which VSMC are exposed to elevated insulin concentrations in the presence of selective inhibition of PI 3-kinase pathway, this beneficial effect of insulin was lost. In an attempt to dissect possible mechanisms for insulin effect on calcification, we found that insulin induces phosphate transport into VSMC. Recently, the kinetic and molecular characteristics of phosphate transport in VSMC have been analyzed in detail, showing that Na-dependent uptake relies exclusively on type III sodium-dependent phosphate co-transporters Pit-1 and Pit-2 (21). Here, we have demonstrated that this is due to an increase in capacity but not affinity for phosphate transport, and may be explained mainly by insulin induction of Pit-2, and to a lesser extent Pit-1.
High ambient phosphate concentrations are known to induce VSMC calcification, a finding which we confirmed. A postulated mechanism is via activation of intracellular signal transduction by inorganic phosphate, causing VSMC to switch to a more osteogenic phenotype and potentiating mineralization of extracellular matrix (22). We found that insulin significantly attenuated the effect of high phosphate on VSMC calcification. Because medial calcification is a risk factor for cardiovascular mortality in type 2 diabetes (1–3), we used an in vitro model of insulin resistance to determine whether insulin’s ability to attenuate VSMC calcification is affected. In a setting of selective impairment of PI 3-kinase pathway signaling and elevated insulin concentrations, insulin no longer attenuates VSMC calcification. Two approaches were used to dissect possible mechanisms for insulin effect on VSMC calcification. One approach utilized PD98059 to inhibit the other major intracellular signaling pathway activated by insulin, the ERK 1/2 MAP kinase pathway. This pathway was also demonstrated to be important for insulin effect to attenuate VSMC calcification, suggesting that intact signaling along both pathways is important to prevent VSMC calcification. Future studies need to address how effects of these pathways are balanced in vivo. The effect of insulin on VSMC calcification has potential implications for calcification in clinical settings of insulin resistance and need to be explored further in vivo in animal models and in humans.
To further examine possible mechanisms for insulin effect on calcification, we turned to insulin effects on phosphate transport in VSMC. The ability of insulin to reduce serum phosphate concentrations and promote the redistribution of phosphate from the circulation into tissues is well-recognized in clinical medicine (23). The most familiar clinical scenario is the lowering of serum phosphate during insulin infusion to treat diabetic ketoacidosis (24). We demonstrated that insulin promotes phosphate transport in VSMC. Our finding that inhibition of the ERK 1/2 MAP kinase pathway but not the PI 3-kinase pathway abolishes the effect of insulin to stimulate VSMC phosphate transport suggests that in the setting of intact ERK 1/2 MAP kinase signaling found in insulin resistance, this effect of insulin on phosphate transport is maintained.
Insulin’s effect to stimulate phosphate transport in VSMC is mediated primarily by an increase in capacity for phosphate transport (Vmax) rather than the affinity for phosphate (Km). The increase in Vmax suggests an increased expression of active transporters in the plasma membrane or possibly an increased pool of transporters, but this was not addressed specifically in the present study. Type III sodium-dependent phosphate (Na/Pi) co-transporters, Pit-1 and Pit-2, are found in tissues throughout the body including VSMC (25), while others (types I, IIa, IIb and IIc) are found in the kidney and other tissues (26), but not in VSMC. We found that insulin induced modest increases in both Pit-1 and Pit-2 protein expression at 24 hr via the ERK 1/2 MAP kinase pathway. In contrast, insulin had only a minimal effect on Pit-1 mRNA and no effect on Pit-2 mRNA expression. The stimulatory effect of insulin on phosphate transport may be partially explained by a transcriptional (Pit-1) and possibly a post-transcriptional (Pit-2) increase in the protein expression of Pit-1 and Pit-2 Na/Pi co-transporters via ERK 1/2 MAP kinase pathway signaling. The finding that ERK 1/2 MAP kinase signaling is important for both insulin induction of phosphate transport and on Pit-1 and Pit-2 expression suggests that these may be related, and in the setting of insulin resistance, these actions of insulin are preserved. Our data suggest an association between insulin-mediated decrease in the extracellular Ca/Pi product and attenuation of calcification, but the roles of intracellular versus extracellular Ca/Pi product need to be investigated in the future studies.
To exclude the possibility that the increased VSMC calcification we observed was due simply to increased cell death, we used a variety of techniques including LDH enzyme activity and caspase-3 enzyme activity, which are increased with cell death, and trypan blue exclusion, which is decreased with cell death. We found that LDH enzyme activity, caspase-3 activity and trypan blue exclusion performed with the appropriate positive and negative controls were not different between control cells and cells incubated with either signaling pathway inhibitors or high phosphate. These results support the notion that the increased VSMC calcification observed with pathway inhibitors and with high phosphate medium is not due merely to decreased VSMC viability. A role for apoptotic bodies in the induction of phosphate-induced vascular calcification has been reported (27,28) but mechanisms for vascular calcification independent of apoptosis have also been demonstrated by other investigators.
There is a clear association between increased vascular calcification and increased cardiovascular mortality in individuals with type 2 diabetes (1–3). Unfortunately, the mechanisms for increased vascular calcification in diabetes mellitus are unknown. However, it is known that individuals with insulin resistance develop compensatory hyperinsulinemia in order to maintain euglycemia. In the setting of insulin resistance, there is selective impairment of insulin signaling (8,18–20) along the PI 3-kinase pathway which mediates the metabolic effects of insulin, insulin stimulation of endothelial nitric oxide synthase expression, and maintenance of VSMC differentiation (9,10,29). These latter potentially anti-atherogenic properties of insulin are likely to be impaired in the setting of insulin resistance. However, insulin signaling along the mitogenic ERK 1/2 MAP kinase pathway remains intact in the setting of metabolic insulin resistance, so hyperinsulinemia could lead to increased signaling along the ERK 1/2 MAP kinase pathway. Since insulin also has mitogenic and proliferative effects which are mediated through this pathway (8,9), these effects may be enhanced in the setting of insulin resistance with concomitant hyperinsulinemia. We employed an in vitro model of selective insulin signaling in insulin resistance using pharmacologic inhibition of the PI 3-kinase pathway and ambient elevated insulin conditions (9). The findings in this study will need to be confirmed in further ex vivo and in vivo studies, particularly since basal PI 3-kinase activity is not reduced in insulin resistance.
In summary, the key inferences that can be made from these studies are: 1) insulin has a protective effect against VSMC calcification in high phosphate conditions, but this effect is lost in the setting of PI 3-kinase pathway impairment, 2) the known effect of insulin to reduce phosphatemia may be explained in part by increased phosphate transport into peripheral tissues, and 3) increased phosphate uptake by VSMC does not correlate with increased extracellular calcification. These observations are potentially quite important because of the selective signaling pathway impairment present in individuals with insulin resistance, particularly type 2 diabetes mellitus and the metabolic syndrome.
Acknowledgments
This work was funded by grants from the Department of Veterans Affairs Office of Research and Development Medical Research Service (CW, ML, BD), the Spanish Minister of Education and Science BFI2003–06645/PR2003–0392 (VS), the Diabetes Trust Foundation (CW), and the National Institutes of Health (ML) Dr. Wang is the recipient of a VA Research Career Development Award. The authors gratefully acknowledge the expert technical microscopy assistance of Ron Bouchard.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lehto S, Niskanen L, Suhonen M, Ronnemaa T, Laakso M. Medial artery calcification: A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16(8):978–83. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 2.Niskanen L, Siitonen O, Suhonen M, Usitupa MI. Medial artery calcification predicts cardiovascular mortality in patients with NIDDM. Diabetes Care. 1994;17(11):1252–6. doi: 10.2337/diacare.17.11.1252. [DOI] [PubMed] [Google Scholar]
- 3.Reaven PD, Sacks J Investigators for the VADT. Coronary artery and abdominal aortic calcification are associated with cardiovascular disease in type 2 diabetes. Diabetologia. 2005;48(2):379–85. doi: 10.1007/s00125-004-1640-z. [DOI] [PubMed] [Google Scholar]
- 4.London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18(9):1731–40. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 5.Doherty TM, Fitzpatrick LA, Inoue D, Qiao J-H, Fishbein MC, Detrano RC, Shah PK, Rajavashisth TB. Molecular, endocrine, and genetic mechanisms of arterial calcification. Endocr Rev. 2004;25(4):629–72. doi: 10.1210/er.2003-0015. [DOI] [PubMed] [Google Scholar]
- 6.Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24(7):1161–70. doi: 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- 7.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Giachelli CM. Phosphate regulation of vascular smooth muscle cells calcification. Circ Res. 2000;87(7):E10–17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 8.Montagnani M, Golovchenko I, Kim I, Koh GY, Goalstone ML, Mundhekar AN, Johansen M, Kucik DF, Quon MJ, Draznin B. Inhibition of phosphatidylinositol 3-kinase enhances mitogenic actions of insulin in endothelial cells. J Biol Chem. 2002;277(3):1794–9. doi: 10.1074/jbc.M103728200. [DOI] [PubMed] [Google Scholar]
- 9.Wang CCL, Gurevich I, Draznin B. Insulin affects vascular smooth muscle cell phenotype and migration via distinct signaling pathways. Diabetes. 2003;52(10):2562–9. doi: 10.2337/diabetes.52.10.2562. [DOI] [PubMed] [Google Scholar]
- 10.Zeng G, Quon MJ. Insulin-stimulated production of nitric oxide is inhibited by wortmannin: direct measurement in vascular endothelial cells. J Clin Investig. 1996;98(4):894–8. doi: 10.1172/JCI118871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Standley PR, Zhang F, Ram JL, Zemel MB, Sowers JR. Insulin attenuates vasopressin-induced calcium transients and a voltage-dependent calcium response in rat vascular smooth muscle cells. J Clin Invest. 1991;88(4):1230–6. doi: 10.1172/JCI115426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito F, Hori MT, Fittingoff M, Hino T, Tuck ML. Insulin attenuates agonist-mediated calcium mobilization in cultured rat vascular smooth muscle cells. J Clin Invest. 1993;92(3):1161–7. doi: 10.1172/JCI116685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuriyama S, Nakamura K, Horiguchi M, Uchida H, Sakai O. Decreased insulin-sensitive Ca2+ transport in cultured vascular smooth muscle cells from spontaneously hypertensive rats. Am J Hypertens. 1992;5(12 Pt 1):892–5. doi: 10.1093/ajh/5.12.892. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan-Albuquerque N, Garat C, Desseva C, Jones PL, Nemenoff RA. Platelet-derived Growth Factor-BB-mediated Activation of Akt suppresses Smooth Muscle-specific Gene Expression through Inhibition of Mitogen-activated Protein Kinase and Redistribution of Serum Response Factor. J Biol Chem. 2003;278:39830–8. doi: 10.1074/jbc.M305991200. [DOI] [PubMed] [Google Scholar]
- 15.Breusegem SY, Halaihel N, Inoue M, Zajicek H, Lederer E, Barry NP, Sorribas V, Levi M. Acute and chronic changes in cholesterol modulate Na-Pi cotransport activity in OK cells. Am J Physiol Renal Physiol. 2005;289(1):F154–65. doi: 10.1152/ajprenal.00331.2004. [DOI] [PubMed] [Google Scholar]
- 16.Sorribas V, Markovich D, Hayes G, Stange G, Forgo J, Biber J, Murer H. Cloning of a Na/Pi cotransporter from opossum kidney cells. J Biol Chem. 1994;269(9):6615–21. [PubMed] [Google Scholar]
- 17.Giuliano AR, Wood RJ. Vitamin D-regulated calcium transport in Caco-2 cells: unique in vitro model. Am J Physiol. 1991;260(2 Pt 1):G207–12. doi: 10.1152/ajpgi.1991.260.2.G207. [DOI] [PubMed] [Google Scholar]
- 18.Jiang ZY, Lin YW, Clemont A, Feener EP, Hein KD, Igarashi M, Yamauchi T, White MF, King GL. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest. 1999;104(4):447–57. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cusi K, Maezono K, Osman A, Pendergrass M, Patti ME, Pratipanawatr T, DeFronzo RA, Kahn CR, Mandarino LJ. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest. 2000;105(3):311–20. doi: 10.1172/JCI7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Book CB, Dunaif A. Selective insulin resistance in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1999;84(9):3110–6. doi: 10.1210/jcem.84.9.6010. [DOI] [PubMed] [Google Scholar]
- 21.Villa-Bellosta R, Bogaert YE, Levi M, Sorribas V. Characterization of phosphate transport in rat vascular smooth muscle cells: implications for vascular calcification. Arterioscler Thromb Vasc Biol. doi: 10.1161/ATVBAHA.106.132266. (in press) [DOI] [PubMed] [Google Scholar]
- 22.Steitz SA, Speer MY, Curinga G, Yang H-Y, Haynes P, Aebersold R, Schinke T, Karsenty G, Giachelli CM. Smooth muscle cell phenotypic transition associated with calcification: Upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res. 2001;89:1147–54. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]
- 23.Harrop GA, Benedict EM. The participation of inorganic substances in carbohydrate metabolism. J Biol Chem. 1924;59:683–97. [Google Scholar]
- 24.Kanter Y, Gerson JR, Bessman AN. 2,3-diphosphoglycerate, nucleotide phosphate, and organic and inorganic phosphate levels during the early phases of diabetic ketoacidosis. Diabetes. 1977;26(5):429–33. doi: 10.2337/diab.26.5.429. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Yang HY, Giachelli CM. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ Res. 2006;98(7):905–12. doi: 10.1161/01.RES.0000216409.20863.e7. [DOI] [PubMed] [Google Scholar]
- 26.Collins JF, Bai L, Ghishan FK. The SLC20 family of proteins: dual functions as sodium-phosphate cotransporters and viral receptors. Pflugers Arch. 2004;447(5):647–52. doi: 10.1007/s00424-003-1088-x. [DOI] [PubMed] [Google Scholar]
- 27.Proudfoot D, Skepper JN, Hegyi L, Bennett MR, Shanahan CM, Weissberg PL. Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circ Res. 2000;87(11):1055–62. doi: 10.1161/01.res.87.11.1055. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL, Shanahan CM. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol. 2004;15(11):2857–67. doi: 10.1097/01.ASN.0000141960.01035.28. [DOI] [PubMed] [Google Scholar]
- 29.Wang CC, Goalstone ML, Draznin B. Molecular mechanisms of insulin resistance that impact cardiovascular biology. Diabetes. 2004;53(11):2735–40. doi: 10.2337/diabetes.53.11.2735. [DOI] [PubMed] [Google Scholar]