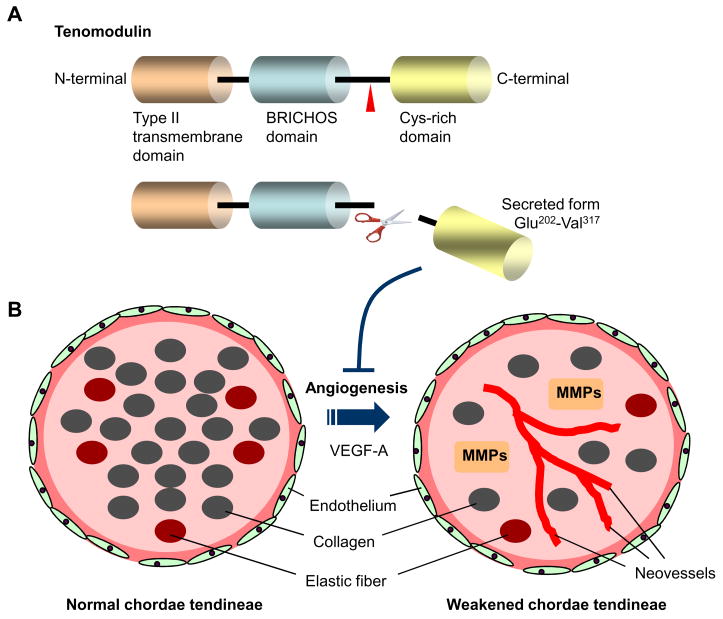
Figure 1.
(A) Schematic structure of human tenomodulin protein. The protein consists mainly of three domains; type II transmembrane domain at the N-terminus (Ile31-Lys51), a BRICHOS domain (Gly93-Ile186), and a cysteine-rich domain at the C-terminus (Phe255-Val317). There are two N-glycosylation sites within the BRICHOS domain, and the potential cleaved site (Arg-Xxx-Xxx-Arg) is located at position 233–236. The cysteine (Cys) rich domain contains eight cysteine residues. The C-terminal domain has a robust anti-angiogenic activity when expressed in a secreted form. (B) Schematic images of normal and pathogenic chordae tendineae. Cross-sectioned chordae show layers of endothelial cells on a basal lamina, the underlying layer consisting of collagen with occasional elastic fibers. Tenomodulin maintains a state of avascularity in healthy chordae tendineae. Loss of tenomodulin is associated with neoangiogenesis, probably mediated by vascular endothelial growth factor (VEGF)-A. Such changes lead to the degenerative process with matrix metalloproteases (MMPs). The reduced material properties can predispose the chordae for mechanical damage or in the long term for spontaneous rupture.