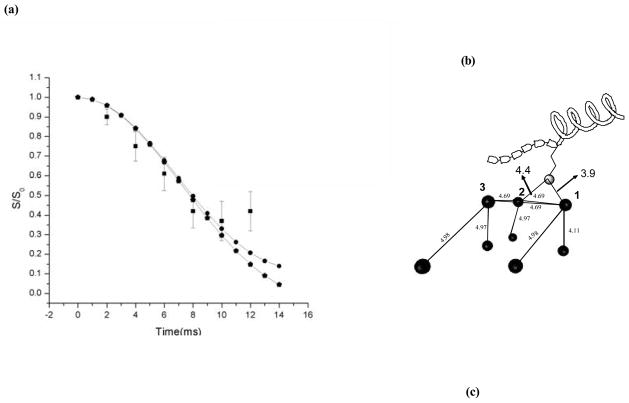
Fig. 9.
(a) Statistical χ2 analysis of 15N-31P REDOR study of binding of 15N K6 of SN-15 to HAP indicates a well-defined minimum distances of 3.8 A and 4.8 A suggesting a possible hydrogen bond between the K6 side-chain and the HAP atoms. The filled squares are the experimental data while the filled circles denote the simulation that gives rise to the χ2 minimum and the pentagons indicate the multiple spin simulation of a 15N K6 approaching the (004) crystal plane of hydroxyapatite (parameters used for the simulation are defined thoroughly in (b)). (b) Model of a 15N K6 sidechain of SN-15 approaching the (004) crystal plane of hydroxyapatite. The figure depicts all the spins that were used the above mentioned simulation. The dark circles denote the spins while the lighter shaded circle depicts the 15N spin on the lysine side-chain. The blocks on the peptide depict the six N-terminal residues of SN-15 that are known to be in an extended conformation.