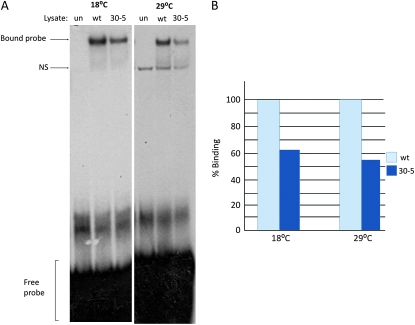
Figure 3.—
DNA binding ability of the 30-5 isoform at 18° and 29°. (A) Unprogrammed lysate (un), wild-type MEF2 (wt), and 30-5 MEF2 proteins were each mixed with a radioactively labeled MEF2 binding site from the enhancer region of Act57B. Reactions and electrophoresis were carried out at both 18° and 29°. These formed complexes that moved considerably slower than free probe through a polyacrylamide gel, indicating that both protein isoforms could bind to the MEF2 recognition site. However, the band intensity was significantly less intense when the 30-5 reaction was compared to wild-type, regardless of temperature. An additional nonspecific band (NS) is seen at 29° in the unprogrammed lysate as well as the experimental lanes. (B) Percentage of binding ability of 30-5 compared to wild type (100%) quantified by band intensity (see materials and methods). Note that the 30-5 isoform consistently bound to DNA less efficiently than wild type.