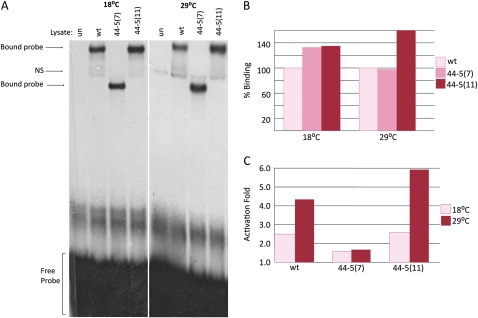
Figure 4.—
DNA binding and activation ability of the 44-5 isoforms. (A) Unprogrammed lysate (un), wild-type MEF2 (wt), and the two 44-5 MEF2 isoforms were each mixed with a radioactively labeled MEF2 binding site from the enhancer region of Act57B and separated on a polyacrylamide gel. Reactions and electrophoresis were carried out at both 18° and 29°. All isoforms of MEF2 were able to bind strongly to the DNA probe; the 44-5 (7) complex is considerably smaller than wild type and 44-5 (11) since the protein is truncated. (B) Percentage of binding ability of 44-5 (7) and (11) compared to wild type (100%) quantified by band intensity. Both isoforms were able to bind to DNA as well as, if not better than, wild type at both temperatures. (C) Activation of a MEF2-dependent Act57B enhancer fused to a lac-Z reporter gene by either wild-type MEF2, 44-5 (7), or 44-5 (11) isoforms in cell culture. The ability of wild-type MEF2 to activate a downstream target gene increased at 29°. 44-5 (7) transactivation ability was not detectable above negative controls at either temperature. 44-5 (11) was able to activate reporter gene expression to a greater degree than wild type.